Abstract
Purpose
To explore the protective effect of probucol on human retinal Müller cells cultured in high glucose.
Methods
Primary Müller cells from human retinas were cultured in complete DMEM. Third-generation Müller cells were identified using glutamine synthetase (GS) antibody and randomly divided into three groups: normoglycemia (NG, 5.5 mmol/L); hyperglycemia (HG, 30 mmol/L); and hyperglycemia (30 mmol/L) with probucol (10 μmol/L; HGPB). After a 24-h intervention, cell proliferation, apoptosis, and cellular reactive oxygen species (ROS) were measured with a CCK-8 kit, flow cytometry, and DCFH-DA probe, respectively. Kelch-like ECH-associated protein 1 (Keap1), NF-E2-related factor 2 (Nrf2), and glutamate cysteine ligase catalytic subunit (GCLC) protein expression were detected by immunofluorescence staining.
Results
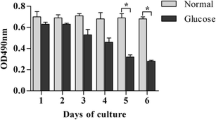
For NG, HG, and HGPB, optical density (OD) values for cell proliferation were 0.98 ± 0.23, 0.58 ± 0.11, and 0.73 ± 0.11; apoptotic rates were 2.79 ± 0.52%, 7.70 ± 0.44%, and 4.00 ± 0.95%; and intracellular ROS were 20.89 ± 5.14, 55.17 ± 14.07, and 26.28 ± 4.73, respectively. Compared to NG, OD was markedly decreased (P < 0.01), apoptosis was increased (P < 0.001), and intracellular ROS level was significantly higher than in HG (P < 0.01). Compared to HG, OD was markedly increased (P < 0.01), apoptosis was meaningfully decreased (P < 0.01), and intracellular ROS level was significantly lower than in HGPB (P < 0.01). GS, Keap1, Nrf2, and GCLC had positive expression.
Conclusions
Probucol could inhibit intracellular ROS generation, promote proliferation, and decrease apoptosis of human retinal Müller cells cultured in high glucose. This might also be associated with Keap1/Nrf2/ARE oxidative stress signaling pathway activation.






Similar content being viewed by others
References
Stitt AW, Curtis TM, Chen M et al (2016) The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res 51(3):156–186
Biedermann B, Bringmann A, Franze K et al (2004) GABA(A) receptors in Müller glial cells of the human retina. Glia 46(3):302–310
Krugel K, Wurm A, Pannicke T et al (2011) Involvement of oxidative stress and mitochondrial dysfunction in the osmotic swelling of retinal glial cells from diabetic rats. Exp Eye Res 92(1):87–93
Wang J, Xu X, Elliott MH et al (2010) Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes 59(9):2297–2305
Zhong Y, Li J, Chen Y et al (2012) Activation of endoplasmic reticulum stress by hyperglycemia is essential for Müller cell-derived inflammatory cytokine production in diabetes. Diabetes 61(2):492–504
Bai Y, Ma JX, Guo J et al (2009) Müller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol 219(4):446–454
Abu El-Asrar AM, Siddiquei MM, Nawaz MI et al (2016) Coexpression of heparanase activity, cathepsin L, tissue factor, tissue factor pathway inhibitor, and MMP-9 in proliferative diabetic retinopathy. Mol Vis 22:424–435
Durning SP, Preston-Hurlburt P, Clark PR et al (2016) The receptor for advanced glycation endproducts drives T cell survival and inflammation in type 1 diabetes mellitus. J Immunol 197(8):3076–3085
Kline CL, Schrufer TL, Jefferson LS et al (2006) Glucosamine-induced phosphorylation of the alpha-subunit of eukaryotic initiation factor 2 is mediated by the protein kinase R-like endoplasmic-reticulum associated kinase. Int J Biochem Cell Biol 38(5–6):1004–1014
Kowluru RA, Mishra M (2015) Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim Biophys Acta 1852(11):2474–2483
Lorenzi M (2007) The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res 2007:61038
Tarr JM, Kaul K, Chopra M et al (2013) Pathophysiology of diabetic retinopathy. ISRN Ophthalmol 2013:343560
Wautier MP, Tessier FJ, Wautier JL (2014) Advanced glycation end products: a risk factor for human health. Ann Pharm Fr 72(6):400–408
Kowluru RA, Kowluru A, Mishra M et al (2015) Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res 48:40–61
Li C, Miao X, Li F et al (2017) Oxidative stress-related mechanisms and antioxidant therapy in diabetic retinopathy. Oxid Med Cell Longev 2017:9702820
Cao Y, Li X, Wang CJ et al (2015) Role of NF-E2-related factor 2 in neuroprotective effect of l-carnitine against high glucose-induced oxidative stress in the retinal ganglion cells. Biomed Pharmacother 69:345–348
Jimenez-Osorio AS, Gonzalez-Reyes S, Pedraza-Chaverri J (2015) Natural Nrf2 activators in diabetes. Clin Chim Acta 448:182–192
Chen B, Lu Y, Chen Y et al (2015) The role of Nrf2 in oxidative stress-induced endothelial injuries. J Endocrinol 225(3):R83–R99
Zhong Q, Mishra M, Kowluru RA (2013) Transcription factor Nrf2-mediated antioxidant defense system in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci 54(6):3941–3948
Satoh T, Mckercher SR, Lipton SA (2013) Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic Biol Med 65:645–657
Xu Z, Wei Y, Gong J et al (2014) NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia 57(1):204–213
Zhou G, Wang Y, He P et al (2013) Probucol inhibited Nox2 expression and attenuated podocyte injury in type 2 diabetic nephropathy of db/db mice. Biol Pharm Bull 36(12):1883–1890
Yang S, Zhao L, Han Y et al (2017) Probucol ameliorates renal injury in diabetic nephropathy by inhibiting the expression of the redox enzyme p66Shc. Redox Biol 13:482–497
Zhang Q, Chen L, Si Z et al (2016) Probucol protects endothelial progenitor cells against oxidized low-density lipoprotein via suppression of reactive oxygen species formation in vivo. Cell Physiol Biochem 39(1):89–101
Zhou Z, Liu C, Chen S et al (2017) Activation of the Nrf2/ARE signaling pathway by probucol contributes to inhibiting inflammation and neuronal apoptosis after spinal cord injury. Oncotarget 8(32):52078–52093
Chen Z-P, Liu J-H, Jiang D-Y et al (2011) Observation of oxidative stress condition and macular edema in patients of early diabetic retinopathy treated by probucol. Zhong Guo Yi Shi Za Zhi 13(2):154–157
Chen Z-P, Tang S-B, Wang Q-C et al (2013) Clinical study of probucol in the treatment of hyperlipidemia diabetic macular edema. Zhong Hua Yan Di Bing Za Zhi 29(5):490–494
Toft-Kehler AK, Skytt DM, Svare A et al (2017) Mitochondrial function in Müller cells—Does it matter? Mitochondrion 36:43–51
Wang JJ, Zhu M, Le YZ (2015) Functions of Müller cell-derived vascular endothelial growth factor in diabetic retinopathy. World J Diabetes 6(5):726–733
Liemburg-Apers DC, Willems PH, Koopman WJ et al (2015) Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch Toxicol 89(8):1209–1226
Han N, Yu L, Song Z et al (2015) Agmatine protects Müller cells from high-concentration glucose-induced cell damage via N-methyl-d-aspartic acid receptor inhibition. Mol Med Rep 12(1):1098–1106
Xi X, Gao L, Hatala DA et al (2005) Chronically elevated glucose-induced apoptosis is mediated by inactivation of Akt in cultured Müller cells. Biochem Biophys Res Commun 326(3):548–553
Fu S, Dong S, Zhu M et al (2015) Müller glia are a major cellular source of survival signals for retinal neurons in diabetes. Diabetes 64(10):3554–3563
Sheng L, Jiao B, Shao L et al (2013) Probucol inhibits hydrogen peroxide to induce apoptosis of vascular smooth muscle cells. Mol Med Rep 7(4):1185–1190
Kowluru RA, Shan Y (2017) Role of oxidative stress in epigenetic modification of MMP-9 promoter in the development of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 255(5):955–962
Wang YY, Li H, Wang XH et al (2016) Probucol inhibits MMP-9 expression through regulating miR-497 in HUVECs and apoE knockout mice. Thromb Res 140:51–58
Katagiri M, Shoji J, Inada N et al (2018) Evaluation of vitreous levels of advanced glycation end products and angiogenic factors as biomarkers for severity of diabetic retinopathy. Int Ophthalmol 38(2):607–615
Mishra M, Zhong Q, Kowluru RA (2014) Epigenetic modifications of Keap1 regulate its interaction with the protective factor Nrf2 in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci 55(11):7256–7265
Du Y, Zhang X, Ji H et al (2012) Probucol and atorvastatin in combination protect rat brains in MCAO model: upregulating Peroxiredoxin2, Foxo3a and Nrf2 expression. Neurosci Lett 509(2):110–115
Duan SB, Liu GL, Wang YH et al (2012) Epithelial-to-mesenchymal transdifferentiation of renal tubular epithelial cell mediated by oxidative stress and intervention effect of probucol in diabetic nephropathy rats. Ren Fail 34(10):1244–1251
Acknowledgements
We thank the staff at the Research Institute of AIER Eye for their assistance in this research and Professor Chang-Luo for his help in writing this paper.
Funding
This study was supported by the Natural Science Foundation Project of Hunan Province (No. 2018JJ2001), China, and the Research Fund Project of AIER Eye Hospital Group (No. AF1601D5), China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, X., Ai, S., Chen, Z. et al. Probucol promotes high glucose-induced proliferation and inhibits apoptosis by reducing reactive oxygen species generation in Müller cells. Int Ophthalmol 39, 2833–2842 (2019). https://doi.org/10.1007/s10792-019-01130-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-019-01130-8