Abstract
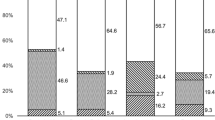
To assess utility of PCR in the diagnosis of bacterial corneal ulcer and to compare sensitivity and specificity of this technique with conventional laboratory methods. A prospective nonrandomized investigative study conducted on 122 eyes of presumed bacterial keratitis. Samples were collected for bacterial and fungal culture and Gram stain smear. A separate sample was taken for PCR with 26 gauge needle and was dipped directly into eppendorff tube with lysis buffer in it. Diagnosis of culture proven bacterial keratitis was established in 53 (43.4 %) and most common bacterial isolate was staphylococcal sp. (83 %). Direct microscopic examination of Gram stained smear revealed presence of bacteria in 24 (23.9 %) specimens and PCR positivity was evident in 56 (45.5 %). In preantibiotic treated eyes culture was positive in 15 (30 %), Gram stain in 9 (18 %), and PCR in 18 (36 %). The same for untreated (fresh) eyes, positivity of culture as well as PCR was noted in 38 (52.7 %) and that of Gram stain was noted in 20 (27.7 %). Sensitivity of Gram stain and PCR was 45.28 and 88.68 % respectively; whereas specificity was 92.75 % for Gram stain and 86.96 % for PCR. The average time taken for PCR reaction was 4–8 h while culture reporting took at least 24–48 h. Our findings suggest that PCR is a good adjunct modality to the “Gold Standard” technique in the diagnosis of bacterial corneal ulcer.




Similar content being viewed by others
References
Whitcher JP et al (1997) Corneal ulceration in developing world–a silent epidemic. Br J Ophthalmol 81:622–623
Brillant LB, Pokhrel RP et al (1985) Epidemiology of blindness in Nepal. Bull World Health Organ 63:375–386
Srinivasan M, Christine AG et al (1997) Epidemiology and etiological diagnosis corneal ulceration in Madurai, south India. Br J Ophthalmol 81:965–971
Dandona R, Dandona L (2003) Corneal blindness in a south Indian population : need for health promotion strategies. Br J Ophthalmol 87:133–141
Dunlop AA, Wright ED et al (1994) Suppurative corneal ulceration in Bangladesh. A study of 142 cases examining the microbiological diagnosis, clinical and epidemiological features of bacterial and fungal keratitis. Aust N Z J Ophthalmol 22:105–110
Satpathy G, Vishalakshi P (1995) Ulcerative keratitis: microbial profile and sensitivity pattern-a five year study. Ann Ophthalmol 27:301–306
Khanal B, Deb M, Panda A et al (2005) Laboratory diagnosis in ulcerative keratitis. Ophthalmic Res 37:123–127
Sharma S, Kunimoto DY, Gopinathan U et al (2002) Evaluation of corneal scraping smear examination methods in the diagnosis of bacterial and fungal keratitis: a survey of eight years of laboratory experience. Cornea 21(7):643–647
Mullis K, Faloona F, Scharf S, Saiki R et al (1986) Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol 51(1):263–273
Rehman DA (1993) The identification of uncultured microbial pathogens. J Infect Dis 168:1–8
Garcia-Ferrer FJ, Blatt AN, Laycock KA et al (1995) Molecular biologic techniques in ophthalmic pathology. Ophthalmol Clin North Am 8:25–36
Haykin PG, Tobal K, McIntyre G et al (1994) The diagnosis of delayed post-operative endophthalmitis by polymerase chain reaction of bacterial DNA in vitreous samples. J Med Microbiol 40:408–415
McCabe KM, Knan G, Zhang YH et al (1995) Amplification of bacterial DNA using highly conserved sequrnces: automated analysis and potential for molecular triage of sepsis. Pediatrics 95:165–169
Katcher HL, Schwartz I et al (1994) A distinctive property of Tth DNA polymerase: enzymatic amplification in the presence of phenol. Biotechniques 16:84–92
Wiedbrank DL, Werner JC et al (1995) Inhibition of PCR by vitreous and aqueous fluids. J Clin Microbiol 33:2643–2646
Dworkic LL, Gibler TM et al (2002) Real time quantitative polymerase chain reaction. Diagnosis of infectious posterior uveitis. Arch Ophthalmol 120:1534–1539
Alderberg JM, Wittwer C (1995) Use of Polymerase chain reaction in the diagnosis of ocular disease. Curr Opin Ophthalmol 6:80–85
Van Galder RN (2001) Application of PCR to diagnosis of ophthalmic diseases. Surv Ophthalmol 46:248–258
Osakabe Y, Yaguchi C, Miyai T et al (2006) Detection of streptococcus species by PCR in infectious crystalline keratopathy. Cornea 25:1227–1230
Vengayil S, Panda A, Satpathy G (2009) Polymerase chain reaction-guided diagnosis of mycotic keratitis: a prospective evaluation of its efficacy and limitations. Invest Ophthalmol Vis Sci 50(1):152–156
Wilson KH (1994) Detection of culture-resistant bacterial pathogens by amplification and sequencing of ribosomal DNA. Clin Infect Dis 18:958–962
Cockerill FR III (1999) Genetic methods for assessing antimicrobial resistance. Antimicrob Agents Chemother 43:199–212
Murakami K, Minamide W, Wada K et al (1991) Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol 29:2240–2244
Khan MA, Ahmad S, Banu N (2010) Molecular characterisation of methicillin-resistant Staphylococcus aureus (MRSA) from keratitis patients: a microbiological analysis. Br J Ophthalmol 94(8):994–998
Satake S, Clark N, Rimland D et al (1997) Detection of vancomycin-resistant enterococci in fecal samples by PCR. J Clin Microbiol 35:2325–2330
Nachamkin I, Kang C, Weinstein MP et al (1997) Detection of resistance to isoniazid, rifampin, and streptomycin in clinical isolates of mycobacterium tuberculosis by molecular methods. Clin Infect Dis 24:894–900
Michele Knox C, Cevellos Vickey (1998) 16S ribosomal DNA typing for identification of pathogens in patients with bacterial keratitis. J Clin Microbiol 36:3492–3496
Inoue T, Ohashi Y (2013) Utility of real-time PCR analysis for appropriate diagnosis for keratitis. Cornea 32(1):S71–S76
Eleinen KG, Mohalhal AA, Elmekawy HE et al (2012) Polymerase chain reaction-guided diagnosis of infective keratitis - a hospital-based study. Curr Eye Res 37(11):1005–1011
Subrayan V, Peyman M, Lek YS et al (2010) Assessment of polymerase chain reaction in the detection of pseudomonas aeruginosa in contact lens-induced severe infectious keratitis. Eye Contact Lens 36(4):201–203
Prabhasawat P, Leelaporn A, Tesavibul N et al (2010) Molecular identification by 16S rDNA sequencing using excised corneal tissues: a useful diagnostic tool for refractory keratitis. Jpn J Ophthalmol 54(1):97–100
Commercial interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panda, A., Pal Singh, T., Satpathy, G. et al. Comparison of polymerase chain reaction and standard microbiological techniques in presumed bacterial corneal ulcers. Int Ophthalmol 35, 159–165 (2015). https://doi.org/10.1007/s10792-014-9925-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-014-9925-9