Abstract
Objective
Curcumin (diferuloylmethane), a major component of turmeric is well known for its anti-inflammatory potential. Present study investigates sequential release of inflammatory mediators post LPS challenge (10 mg/kg,i.p.) causing lung inflammation and its modulation by curcumin through different routes (20 mg/kg, i.p and 10 mg/kg, i.n.) in murine model. Dexamethasone (1 mg/kg, i.p) was used as standard drug.
Methods
Lung Inflammation was evaluated by histopathological analysis, myeloperoxidase (MPO) activity followed by inflammatory cell count and total protein content measurements in bronchoalveolar fluid (BALF). Reactive oxygen species (ROS), nitrite and TNF-α levels were measured as markers of endotoxin shock at different time points (1–72 h). The mRNA expression of transforming growth factors-β1 (TGF-β1), iNOS and Toll-like receptor-4 (TLR-4) were measured followed by Masson’s trichrome staining and hydroxyproline levels as collagen deposition marker leading to fibrotic changes in lungs.
Results
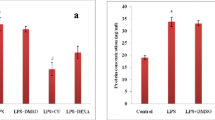
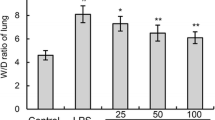
We found that LPS-induced lung inflammation and injury was maximum 24-h post LPS challenge shown by MPO and histological analysis which was further supported by elevated nitrite and ROS levels whereas TNF-α level was highest after 1 h. Endotoxin-induced mortality was significantly reduced in curcumin (i.p) pretreatment groups up to 72-h post LPS challenge. Significant inhibition in mRNA expression of iNOS, TGF-β1 and TNF-α level was noted after curcumin treatment along with lowered MPO activity, inflammatory cell count, ROS, nitrite levels and collagen deposition in lungs.
Conclusion
Our results suggest that higher endotoxin dose causes inflammatory mediator release in chronological order which tend to increase with time and reached maximum after 24-h post-endotoxin (LPS) exposure. Intraperitoneal route of curcumin administration was better in modulating inflammatory mediator release in early phase as compared to intranasal route of administration. It can be used as supplementary therapeutic intervention at early stage of endotoxemia, having fewer side effects.










Similar content being viewed by others
References
Abe Y, Shu Hashimoto, Horie T (1999) Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res 39:41–47
Andonegui G, Bonder CS, Green F (2003) Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J Clin Investig 111:1011–1120
Bartko J, Stiebellehner L, Derhaschnig U, Schoergenhofer C, Schwameis M, Prosch H, Jilma B (2016) Dissociation between systemic and pulmonary anti-inflammatory effects of dexamethasone in humans. Brit J Pharmacol. doi:10.1111/bcp.12857
Bogdan C, Nathan C (1993) Modulation of macrophage function by transforming growth factor β, interleukin-4, and interleukin-10a. Ann NY Acad Sci 685:713–739
Chen Y, Kam CS, Liu FQ (2008) LPS-induced up-regulation of TGF-β receptor 1 is associated with TNF-α expression in human monocyte-derived macrophages. J Leukoc Biol 83:1165–1173
Craciun FL, Schuller ER, Remick DG (2010) Early enhanced local neutrophil recruitment in peritonitis-induced sepsis improves bacterial clearance and survival. J Immunol 185:6930–6938
Eachempati SR, Hydo LJ, Shou J (2007) Outcomes of acute respiratory distress syndrome (ARDS) in elderly patients. J Trauma Acute Care Surg 63:344–350
Eruslanov E, Kusmartsev S (2010) Identification of ROS using oxidized DCFDA and flow-cytometry. Adv Protoc Oxid Stress 2:57–72
Fein AM, Calalang-Colucci MG (2000) Acute lung injury and acute respiratory distress syndrome in sepsis and septic shock. Crit Care Clin 16:289–317
Garbe E, LeLorier J, Boivin JF, Suissa S (1997) Inhaled and nasal glucocorticoids and the risks of ocular hypertension or open-angle glaucoma. JAMA 277:722–727
Giulietti A, Overbergh L, Valckx D (2001) An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25:386–401
Grammes J, Soehnlein O (2011) Contribution of neutrophils to acute lung injury. Mol Med 17:293–307
Gunaydın M, Guzel A (2012) The effect of curcumin on lung injuries in a rat model induced by aspirating gastrointestinal decontamination agents. J Pediatr Surg Case 47:1669–1676
Gupta SC, Patchva S, Koh W (2012) Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol 39:283–299
Hattori N, Degen JL, Sisson TH (2000) Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Investig 106:1341–1350
He Z, Chen X, Wang S (2014) Toll–like receptor-4 monoclonal antibody attenuates lipopolysaccharide–induced acute lung injury in mice. Exp Ther Med 8(3):871–876
Hotchkiss RS, Monneret G, Payen D (2013) Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13:260–268
Kaymak C, Basar H, Sardas S (2011) Reactive oxygen species (ROS) generation in sepsis. FABAD J Pharm Sci 36:41–70
Kim JJ, Lee SB, Park JK, Yoo YD (2010) TNF-α-induced ROS production triggering apoptosis is directly linked to Romo1 and Bcl-xl. Cell Death Differ 17:1420–1434
Kumari A, Tyagi N, Dash D (2015) Intranasal curcumin ameliorates lipopolysaccharide-induced acute lung injury in mice. Inflammation 38:1103–1112
Lee KY, Ho SC, Lin HC (2006) Neutrophil-derived elastase induces TGF-β1 secretion in human airway smooth muscle via NF-κB pathway. Am J Respir Cell Mol 35:407–414
Madan B, Ghosh B (2003) Diferuloylmethane inhibits neutrophil infiltration and improves survival of mice in high-dose endotoxin shock. Shock 19:91–96
Mayr FB, Yende S, Angus DC (2014) Epidemiology of severe sepsis. Virulence 5:4–11
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20:1126–1167
Parameswaran N, Patial S (2010) Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr 20:87–103
Patel GP, Balk RA (2012) Systemic steroids in severe sepsis and septic shock. Am Res Crit Care Med 185:133–139
Pellacani A, Wiesel P, Razavi S (2001) Down-regulation of high mobility group-I (Y) protein contributes to the inhibition of nitric-oxide synthase 2 by transforming growth factor-β1. J Biol Chem 276:1653–1659
Perros F, Lambrecht BN, Hammad H (2011) TLR4 signaling in pulmonary stromal cells is critical for inflammation and immunity in the airways. Respir Res 12:125–130
Phan SH (2012) Genesis of the myofibroblast in lung injury and fibrosis. Proc Am Thorac Soc 9:148–152
Reddy RC, Chen G, Tekchandani PK (2000) Sepsis-induced immunosuppression: from bad to worse. Immunol Res 24:273–287
Renshaw M, Rockwell J, Engleman C (2002) Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol 169:4697–4701
Reutershan J, Basit A, Galkina EV, Ley K (2005) Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 289:807–815
Schulte W, Bernhagen J, Bucala R (2013) Cytokines in sepsis: potent immunoregulators and potential therapeutic targets-an updated view. Mediat Inflamm. doi:10.1155/2013/165974
Sheridan BC, McIntyre RC, Moore EE (1997) Neutrophils mediate pulmonary vasomotor dysfunction in endotoxin-induced acute lung injury. J Trauma Acute Care Surg 42:391–397
Smith MR, Gangireddy SR, Narala VR (2010) Curcumin inhibits fibrosis-related effects in IPF fibroblasts and in mice following bleomycin-induced lung injury. Am J Physiol Lung Cell Mol Physiol 298:616–625
Sompamit K, Kukongviriyapan U, Nakmareong S (2009) Curcumin improves vascular function and alleviates oxidative stress in non-lethal lipopolysaccharide-induced endotoxemia in mice. Eur J Pharmacol 616:192–199
Tsujimoto H, Ono S, Majima T (2005) Neutrophil elastase, MIP-2, and TLR-4 expression during human and experimental sepsis. Shock 23:39–44
Tyagi N, Kumari A, Dash D (2014) Protective effects of intranasal curcumin on paraquot induced acute lung injury (ALI) in mice. Environ Toxicol Pharmacol 38:913–921
Victor VM, De la Fuente M (2003) Several functions of immune cells in mice changed by oxidative stress caused by endotoxin. Physiol Res 52:789–796
Xiao X, Yang M, Sun D (2012) Curcumin protects against sepsis-induced acute lung injury in rats. J Surg Res 176:31–39
Xu F, Lin SH, Yang YZ (2013) The effect of curcumin on sepsis-induced acute lung injury in a rat model through the inhibition of the TGF-β1/SMAD3 pathway. Int Immunopharmacol 16:1–6
You HJ, Choi CY, Jeon YJ, Chung YC, Kang SK, Hahm KS, Jeong HG (2002) Suppression of inducible nitric oxide synthase and tumor necrosis factor-α expression by 4-nonylphenol in macrophages. Biochem Biophys Res Commun 294:753–759
Acknowledgements
Authors are thankful to the Department of Science and Technology-Science and Engineering Research Board, India (DST-SERB) Project No. (P-07/526) for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kumari, A., Dash, D. & Singh, R. Curcumin inhibits lipopolysaccharide (LPS)-induced endotoxemia and airway inflammation through modulation of sequential release of inflammatory mediators (TNF-α and TGF-β1) in murine model. Inflammopharmacol 25, 329–341 (2017). https://doi.org/10.1007/s10787-017-0334-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0334-3