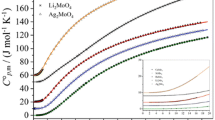
Abstract
Knowledge of the fusion enthalpy as a function of temperature is necessary when performing rigorous thermodynamic calculations involving crystal-liquid transition. The fusion enthalpy can be measured directly only at the melting point (Tm). Derivation of its temperature dependence according to Kirchhoff’s law of Thermochemistry requires measuring the heat capacities of liquid and solid in a temperature range of interest, which is obstructed by fast crystallization of the liquids below the melting temperature. Another option is an extrapolation of the temperature dependence of the melt heat capacity. Its precise measurement in a wide range demands remarkable efforts, especially in the case of high-melting and thermally unstable compounds. In this work, we showed that the fusion enthalpy between 298.15 K and Tm can be estimated using two relatively simple experiments: solution calorimetry and conventional fusion enthalpy measurement at Tm. The fusion enthalpies interpolated as a linear function of temperature were compared with the values derived according to Kirchhoff’s law of Thermochemistry. An agreement within 1–2 kJ·mol−1 was observed for the vast majority of compounds.


Similar content being viewed by others
References
C. Held, J. Brinkmann, A.-D. Schröder, M.I. Yagofarov, S.P. Verevkin, Fluid Phase Equilib. 455, 43 (2018)
Y.Z. Chua, H.T. Do, C. Schick, D. Zaitsau, C. Held, RSC Adv. 8, 6365 (2018)
J.D. Hoffman, J. Chem. Phys. 29, 1192 (1958)
A. Rojas, E. Orozco, Thermochim. Acta 405, 93 (2003)
J.S. Chickos, S. Hosseini, D.G. Hesse, J.F. Liebman, Struct. Chem. 4, 271 (1993)
Y. Kong, J. Hay, Eur. Polymer J. 39, 1721 (2003)
Y. Kong, J. Hay, Polymer 43, 3873 (2002)
J.R. Donnelly, L.A. Drewes, R.L. Johnson, W.D. Munslow, K.K. Knapp, G.W. Sovocool, Thermochim. Acta 167, 155 (1990)
J.P. McCullough, G. Waddington, Anal. Chim. Acta 17, 80 (1957)
S.H. Neau, S.V. Bhandarkar, E.W. Hellmuth, Pharm. Res. 14, 601 (1997)
H. Hojjati, S. Rohani, Org. Proc. Res. Dev. 10, 1110 (2006)
C. Huang, Z. Chen, Y. Gui, C. Shi, G.G. Zhang, L. Yu, J. Chem. Phys. 149, 054503 (2018)
M.I. Yagofarov, S.E. Lapuk, T.A. Mukhametzyanov, M.A. Ziganshin, C. Schick, B.N. Solomonov, Thermochim. Acta 668, 96 (2018)
D.N. Bolmatenkov, M.I. Yagofarov, T.A. Mukhametzyanov, M.A. Ziganshin, C. Schick, B.N. Solomonov, Thermochim. Acta, 178805 (2020)
A. Abdelaziz, D. Zaitsau, T. Mukhametzyanov, B. Solomonov, P. Cebe, S. Verevkin, C. Schick, Thermochim. Acta 657, 47 (2017)
P. Cebe, B.P. Partlow, D.L. Kaplan, A. Wurm, E. Zhuravlev, C. Schick, Thermochim. Acta 615, 8 (2015)
H.T. Do, Y.Z. Chua, A. Kumar, D. Pabsch, M. Hallermann, D. Zaitsau, C. Schick, C. Held, RSC Adv. 10, 44205 (2020)
D.N. Bolmatenkov, M.I. Yagofarov, T.A. Mukhametzyanov, M.A. Ziganshin, B.N. Solomonov, Thermochim. Acta 693, 178778 (2020)
T. Mahnel, V. Štejfa, M. Fulem, K. Růžička, Fluid Phase Equilib. 434, 74 (2017)
M. Fulem, V. Laštovka, M. Straka, K. Růžička, J.M. Shaw, J. Chem. Eng. Data 53, 2175 (2008). https://doi.org/10.1021/je800382b
P. Goursot, H.L. Girdhar, E.F. Westrum Jr., J. Phys. Chem. 74, 2538 (1970)
N. Durupt, A. Aoulmi, M. Bouroukba, M. Rogalski, Thermochim. Acta 260, 87 (1995)
S.S. Chang, J. Chem. Phys. 79, 6229 (1983)
Y. Grosu, L. González-Fernández, U. Nithiyanantham, A. Faik, Energies 12, 3765 (2019)
U. Domanska, D. Wyrzykowska-Stankiewicz, Thermochim. Acta 179, 265 (1991)
M.I. Yagofarov, R.N. Nagrimanov, B.N. Solomonov, J. Mol. Liq. 256, 58 (2018)
M.I. Yagofarov, R.N. Nagrimanov, M.A. Ziganshin, B.N. Solomonov, J. Chem. Thermodyn. 120, 21 (2018)
M.I. Yagofarov, R.N. Nagrimanov, M.A. Ziganshin, B.N. Solomonov, J. Chem. Thermodyn. 116, 152 (2018)
B.N. Solomonov, M.I. Yagofarov, J. Mol. Liq. 319, 114330 (2020)
M.I. Yagofarov, S.E. Lapuk, T.A. Mukhametzyanov, M.A. Ziganshin, T.F. Valiakhmetov, B.N. Solomonov, J. Chem. Thermodyn. 137, 43 (2019)
B.N. Solomonov, M.I. Yagofarov, R.N. Nagrimanov, J. Mol. Liq. 342, 117472 (2021)
W.-K. Wong, E.F. Westrum Jr., Mol. Cryst. Liq. Cryst. 61, 207 (1980)
R.D. Chirico, S. Knipmeyer, W. Steele, J. Chem. Thermodyn. 34(11), 1885 (2002)
M. Hanaya, T. Hikima, M. Hatase, M. Oguni, J. Chem. Thermodyn. 34, 1173 (2002)
C. De Kruif, J. Van Miltenburg, J. Blok, J. Chem. Thermodyn. 15, 129 (1983)
W. Acree Jr., J.S. Chickos, J. Phys. Chem. Ref. Data 46, 013104 (2017)
M.I. Yagofarov, B.N. Solomonov, J. Chem. Thermodyn. 152, 106278 (2021)
M.I. Yagofarov, S.E. Lapuk, T.A. Mukhametzyanov, M.A. Ziganshin, C. Schick, B.N. Solomonov, J. Mol. Liq. 278, 394 (2019)
H. Finke, J. Messerly, S. Lee, A. Osborn, D. Douslin, J. Chem. Thermodyn. 9, 937 (1977)
P.R. van der Linde, J.C. van Miltenburg, G.J. van den Berg, H.A. Oonk, J. Chem. Eng. Data 50, 164 (2005)
J. Cees van Miltenburg, H.A.J. Oonk, G.J.K. van den Berg, J. Chem. Eng. Data 45, 704 (2000)
I. Rabinovich, N. Karyakin, E. Dzharimova, S. Siling, I. Ponomarev, S. Vinogradova, Bull. Acad. Sci. USSR, Div. Chem. Sci. 33, 716 (1984)
B.N. Solomonov, M.A. Varfolomeev, R.N. Nagrimanov, V.B. Novikov, D.H. Zaitsau, S.P. Verevkin, Thermochim. Acta 589, 164 (2014)
B.N. Solomonov, M.A. Varfolomeev, R.N. Nagrimanov, V.B. Novikov, M.A. Ziganshin, A.V. Gerasimov, S.P. Verevkin, J. Chem. Eng. Data 60, 748 (2015)
R.N. Nagrimanov, A.A. Samatov, A.V. Buzyurov, A.G. Kurshev, M.A. Ziganshin, D.H. Zaitsau, B.N. Solomonov, Thermochim. Acta 668, 152 (2018)
Acknowledgements
This work was supported by the Russian Science Foundation (Project No. 22-43-04412).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yagofarov, M.I., Solomonov, B.N. Interpolation of the Temperature Dependence of the Fusion Enthalpy of Aromatic Compounds Between 298.15 K and the Melting Temperature. Int J Thermophys 43, 90 (2022). https://doi.org/10.1007/s10765-022-03020-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-022-03020-1