Abstract
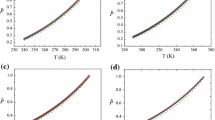
It is shown that the “meso-phase” hypothesis of Woodcock L. V. fails to describe quantitatively and qualitatively the isochoric and isobaric heat capacities, speed of sound, long wavelength limit of the structural factor, isothermal compressibility, density fluctuations, Joule–Thompson coefficient and isothermal throttling coefficient of argon in the “meso-phase” region. It is also shown that VdW-EOS can describe qualitatively the excess Gibbs energy and rigidity of argon near-critical point.






Similar content being viewed by others
References
M.A. Anisimov, Critical Phenomena in Liquids and Liquid Crystals (Gordon and Breach, New York, 1991)
R.J. Baxter, Exactly Solved Models in Statistical Mechanics (Academic Press, London, 1982)
A. Pelissetto, E. Vicari, Critical phenomena and renormalization-group theory. Phys. Rep. 368, 549 (2002). https://doi.org/10.1016/S0370-1573(02)00219-3
J.V. Sengers, M.A. Anisimov, Comment Gibbs density surface of fluid argon: revised critical parameters, L. V. Woodcock. Int. J. Thermophys. (2014) 35, 1770–1784. Int. J. Thermophys. 36, 3001 (2015). https://doi.org/10.1007/s10765-015-1954-8
J.M.H. Levelt Sengers, Liquidons and gasons; controversies about continuity of states. Phys. A 98, 363 (1979). https://doi.org/10.1016/0378-4371(79)90145-6
J.D. van der Waals, On the continuity of gas and liquid states, (original Ph.D. Thesis Leiden 1873) translated and edited by J. S. Rowlinson. (Dover Inc., New York 1987)
J.O. Hirschfelder, C.F. Curtiss, R.B. Bird, Molecular theory of gases and liquids (Wiley, London, 1954), p. 930
L.D. Landau, E.M. Lifshitz, Statistical physics, 3rd edn. (Butterworth-Heinemann, Oxford, 2013)
S.M. Walas, Phase equilibria in chemical engineering. Parts 1 and 2 (Butterworth publishers, London, 1985)
R.C. Reid, J.M. Prausnitz, T.K. Sherwood, The properties of gases and liquids (McGraw-Hill Inc., London, 1977)
R. Balesku, Equilibrium and non-equilibrium statistical mechanics (Wiley, New York, 1975), p. 405
D.Y. Ivanov, Critical Behavior of Non-Ideal Systems (WILEY-VCH Verlag GmbH & Co KGaA, Weinheim, 2008)
V.A. Rabinovich, V.V. Sychev, YuE Sheludyak, The regularities of the thermodynamics of critical phenomena in the liquid-gas system. High Temp. 33(2), 293–315 (1995)
NIST Thermo-physical Properties of Fluid Systems (2017), http://webbook.nist.gov/chemistry/fluid/
M.I. Bagatskii, A.V. Voronel, V.G. Gusak, Zh Exp, Teor. Fiz. Sov. Phys. JETP 43, 728 (1962)
M.I. Bagatskii, A.V. Voronel, V.G. Gusak, Sov. Phys. JETP 16, 517 (1963)
C. Tegeler, R. Span, W. Wagner, A new equation of state for argon covering the fluid region for temperatures from the melting line to 700 K at pressures up to 1000 MPa. J. Phys. Chem. Ref. Data 28(3), 779–850 (1999)
R. Span, W. Wagner, Equations of state for technical applications. II. Results for nonpolar fluids. Int. J. Thermophys. 24, 41 (2003)
R. Span, W. Wagner, Equations of state for technical applications. III. Results for polar fluids. Int. J. Thermophys. 24, 111 (2003)
E.W. Lemmon, R. Span, Short fundamental equations of state for 20 industrial fluids. J. Chem. Eng. Data 51, 785 (2006)
R. Gilgen, R. Kleinrahm, W. Wagner, Measurement and correlation of the (pressure-density-temperature) relation of argon I. The homogeneous gas and liquid regions in the temperature range from 90 K to 340 K at pressures up to 12 MPA. J. Chem. Thermodyn. 26, 383 (1994)
R. Gilgen, R. Kleinrahm, W. Wagner, Measurement and correlation of the (pressure-density-temperature) relation of argon II. Saturated-liquid and saturated-vapor densities and vapour pressures along the entire coexistence curve. J. Chem. Thermodyn. 26, 399 (1994)
M.N. Berberan-Santos, E.N. Bodunov, L. Pogliani, The van der Waals equation: analytical and approximate solutions. J. Math. Chem. 43, 1437–1457 (2008)
D.C. Johnston. Advances in Thermodynamics of the van der Waals Fluid. David C. Johnston. 118 pp. IOP Concise Physics, Morgan & Claypool, San Rafael, CA (2014)
D.C. Johnston. Thermodynamic Properties of the van der Waals Fluid. arXiv preprint arXiv:1402.1205 (2014)
L.V. Woodcock, Gibbs density surface of fluid argon: revised critical parameters. Int. J. Thermophys. 35, 1770 (2014). https://doi.org/10.1007/s10765-013-1411-5
I.H. Umirzakov, New comment on Gibbs density surface of fluid argon: revised Critical Parameters, L. V. Woodcock, Int. J. Thermophys. (2014) 35, 1770–1784. Int. J. Thermophys. 39, 8 (2018). https://doi.org/10.1007/s10765-017-2331-6
L.V. Woodcock, On the interpretation of near-critical gas–liquid heat capacities. Int. J. Thermophys. 38, 139 (2017). https://doi.org/10.1007/s10765-017-2277-8
L.V. Woodcock, Failures of van der Waals’ Equation at the Gas-Liquid Critical Point. Int. J. Thermophys. 39, 120 (2018). https://doi.org/10.1007/s10765-018-2444-6
I.H. Umirzakov, On the Interpretation of Near-Critical Gas–Liquid Heat Capacities by L. V. Woodcock, Int. J. Thermophys. (2017) 38, 139: comments. Int. J. Thermophys. 39, 139 (2018). https://doi.org/10.1007/s10765-018-2414-z
A.G. Stoletov, Collected Works, in 4 volumes (Gostekhteorizdat, Moscow, Leningrad, 1939) 1, 276, 307, 333
M.A. Weinberger, W.G. Schneider, On the liquid-vapor coexistence curve of xenon in the region of the critical temperature. Can. J. Chem. 30, 422 (1952)
M.A. Weinberger, W.G. Schneider, On the liquid-vapor coexistence curve of xenon in the region of the critical temperature. Can. J. Chem. 30, 815 (1952)
M.A. Weinberger, W.G. Schneider, Density distributions in a vertical tube containing xenon near the critical temperature as measured by a radioactive tracer technique. Can. J. Chem. 30, 847 (1952)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Let us consider the first assertion “In contrast to the conjecture [1] there is no reliable experimental evidence to doubt the existence of a single critical point,” citing the Sengers and Anisimov comment [2] based upon historic evidence from divergent isochoric heat capacity \( C_{V} \) measurements at the critical temperature (\( T_{c} \)) which was discussed in [29].
The first part of the assertion (“In contrast to the conjecture [1] there is no reliable experimental evidence to doubt the existence of a single critical point”) was quoted from [4] in [27], but there was not the rest part of the assertion (“citing the Sengers and Anisimov comment [2] based upon historic evidence from divergent isochoric heat capacity \( C_{V} \) measurements at the critical temperature (\( T_{c} \))”) in [27]. The first part of the assertion means that there is no reliable experimental evidence to doubt the existence of a single critical point and this is in contrast to the conjecture of [29] and nothing more. So, the first assertion discussed in [29] is an incorrect assertion from [27], while a correct assertion from [27] is:
Assertion 1
“In contrast to the conjecture [1] there is no reliable experimental evidence to doubt the existence of a single critical point.”
It is evident that Assertion 1 does not mean that Anisimov and Sengers divergent \( C_{V} \) at \( T_{c} \) is wrong. Therefore, the conclusions “if Umirzakov’s first assertion were to be right, Anisimov and Sengers divergent \( C_{V} \) at \( T_{c} \) would have to be wrong. In fact, neither of the assertions will withstand scientific scrutiny” [29] have no sense.
The second assertion discussed in [29] is “… to prove that the existence of a single critical point of a fluid described by van der Waals equation of state (VDW-EOS) is not a hypothesis and is a consequence of the thermodynamic conditions of liquid–vapor phase equilibrium.”
One can see from [27] that the quote in the second assertion is incorrect and a correct assertion from [27] is:
Assertion 2
“We prove that the existence of a single critical point of a fluid described by van der Waals equation of state (VDW-EOS) is not a hypothesis and is a consequence of the thermodynamic conditions of liquid–vapor phase equilibrium.”
It is easy to see reading [29] that there is no proof in [29] that the existence of a single critical point of the fluid described by VdW-EOS is hypothetical and the existence of a single critical point of VdW-fluid is not a consequence of the thermodynamic conditions of liquid–vapor phase equilibrium. So, there is no proof in [29] that Assertion 2 is incorrect.
One can see that VdW-EOS [6] alone was considered in [27] and all conclusions of [27] concern VdW-fluid. There is no statement or assumption in [27] that VDW-EOS describes quantitatively the thermodynamic properties of the real fluids. It is evident that the statements of [27] that “there is no reliable experimental evidence to doubt the existence of a single critical point” and “the existence of a single critical point of a fluid described by the van der Waals equation of state (VDW-EOS) is not a hypothesis and is a consequence of the thermodynamic conditions of liquid–vapor phase equilibrium” do not mean that VdW-EOS describes quantitatively the thermodynamic properties of the real fluids (for example, of argon). It is also evident that the proof that VdW-EOS cannot describe quantitatively the thermodynamic properties of the real fluids does not mean that the above statements of [27] are incorrect.
According to [29], “it was incorrectly asserted that van der Waals equation ‘proves’ the existence of a scaling singularity with a divergent isochoric heat capacity (\( C_{V} \))” in [27]. One can easily see from [27] that there is no assertion in [27] that VdW-EOS proves the existence of a scaling singularity with a divergent isochoric heat capacity.
One can see from the above comments that there is the lack of logic in the reasoning of Woodcock in [29].
We proved in [27] that VdW-fluid has only one critical point. Therefore, the statement “Ref. [1] proves nothing more than van der Waals’ equation has a singularity with two vanishing derivatives” [29] is incorrect if the singularity does not mean that there is only one critical point.
The ability of VdW-EOS to describe the thermodynamic properties of real fluid was not considered in [27]. The fact that VdW-EOS cannot describe quantitatively the thermodynamic properties of the real fluids was earlier established by many authors [7,8,9, 23]. So, the statement in [29] that “state functions of van der Waal’s equation fail to describe the thermodynamic properties of low-temperature gases, liquids and gas–liquid coexistence” is not a new insight into the science or physics.
Many conclusions in [29] are based on the fact that VdW-EOS cannot describe quantitatively the thermodynamic properties of the real fluids. This fact does not prove the statements such as “The conclusion that there is no ‘critical point’ singularity on Gibbs density surface remains scientifically sound,” “the conclusion in Ref. [1], i.e., that there is no critical point singularity with scaling properties on Gibbs density surface still holds true,” and “Van der Waals hypothetical singular critical point is based upon a common misconception that van der Waals equation represents physical reality of fluids” [29].
According to [29] “Explicitly built into the equation is an incorrect a priori assumption of continuity of liquid and gaseous states.” One can see from the detailed consideration of [29] that there is no proof of the incorrectness of a priori assumption of continuity of liquid and gaseous states in [29].
There exists the method for direct experimental measure of a critical density—the disappearance of the meniscus method which gives a high precision of the critical density determination (± 0.02 %) [12, 31,32,33]. The radioactive tracer technique is also used for direct measurement of the critical density [34]. So, the statement “No research in history has reported the direct experimental measurement of a critical density” [29] is incorrect.
One can see from comparison of contents of [27] and [29] that [29] does not include the proofs of the incorrectness of the assertions and conclusions made in [27]. One can also see the same from the comments presented above.
Rights and permissions
About this article
Cite this article
Umirzakov, I.H. Failures of “Meso-Phase” Hypothesis Near Vapor–Liquid Critical Point. Int J Thermophys 40, 21 (2019). https://doi.org/10.1007/s10765-019-2483-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-019-2483-7