Abstract
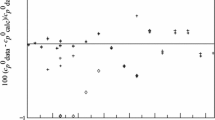
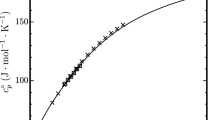
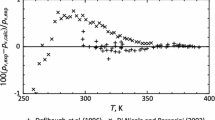
Fluoroethane (R161, C2H5F, 353-36-6) is a potential alternative refrigerant with excellent cycle performance, with zero ozone-depletion potential and low global warming potential. In this study, the thermodynamic property formulation for fluoroethane has been developed with the use of available experimental thermodynamic property data. In determining the equation of state, multiproperty fitting methods were used including single-phase pressure–density–temperature (pρT), vapor pressure, and saturated liquid-density data. The equation of state has been developed to conform to the Maxwell criterion for two-phase liquid–vapor equilibrium states, and is valid for temperatures from 130 K to 450 K, and pressures to 5 MPa. The extrapolation behavior of the equation of state at high temperatures and high pressures is reasonable. As there are very few compressed liquid-density experimental data published, the uncertainties in density of the equation of state are estimated to be 2.0 % in the compressed-liquid region and 0.5 % in the gas and supercritical regions. Uncertainties in vapor pressure are 0.5 % above 200 K and increase at lower temperatures. The uncertainties for all properties are higher in the critical region, except vapor pressure. Detailed comparisons between experimental and calculated data have been performed in this study.
Similar content being viewed by others
Abbreviations
- a :
-
Specific Helmholtz energy
- a i , c i , v i , n i :
-
Coefficients
- B :
-
Second virial coefficient
- C :
-
Third virial coefficient
- c p :
-
Specific isobaric heat capacity
- c v :
-
Specific isochoric heat capacity
- \({d_{i}, l_{i}, t_{i}, \eta_{i}, \beta_{i}, \gamma_{i},\varepsilon_{i}, u_{i}}\) :
-
Exponents
- h :
-
Specific enthalpy
- i :
-
Index number
- M :
-
Molar mass
- N :
-
Number of data points
- p :
-
Pressure
- R :
-
Molar gas constant
- s :
-
Specific entropy
- T :
-
Temperature
- w :
-
Sound speed
- Δ:
-
Deviation
- α :
-
Dimensionless Helmholtz energy
- δ :
-
Reduced density
- ρ :
-
Density
- τ :
-
Inverse reduced temperature
- ω :
-
Acentric factor
- 0:
-
Ideal gas
- r:
-
Residual
- ′:
-
Saturated-liquid state
- 0:
-
Reference state
- c:
-
Critical
- calc:
-
Calculated
- esd:
-
Estimated
- exp:
-
Experimental
- l:
-
Liquid
- nbp:
-
Normal boiling point
- v:
-
Vapor
- σ :
-
Saturation
References
Cui X.I., Chen G.M., Han X.H., Wang Q.: Fluid Phase Equilib. 245, 155 (2006)
Beyerlein A.L., DesMarteau D.D., Kul I., Zhao G.: Fluid Phase Equilib. 150, 287 (1998)
Booth H.S., Swinehart C.F.: J. Am. Chem. Soc. 57, 1337 (1935)
Span R.: Multiparameter Equations of State - An Accurate Source of Thermodynamic Property Data. Springer, Berlin (2000)
M. Frenkel, V. Diky, C.D. Muzny, A.F. Kazakov, J.W. Magee, I.M. Abdulagatov, J.W. Kang, NIST ThermoData Engine, NIST Standard Reference Database 103b, Version 5.0 (Standard Reference Data Program, National Institute of Standards and Technology, Gaithersburg, MD, 2010)
B.E. Poling, J.M. Prausnitz, J.P. O’Connell, The Properties of Gases and Liquids, 5th edn. (McGraw-Hill, New York, 2000)
E.W. Lemmon, M.L. Huber, M.O. McLinden, NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version8.0 (Standard Reference Data Program, National Institute of Standards and Technology, Gaithersburg, MD, 2007)
Lemmon E.W., Jacobsen R.T.: J. Phys. Chem. Ref. Data 34, 68 (2005)
Wagner W., Prüss A.: J. Phys. Chem. Ref. Data 31, 387 (2002)
Grosse A.V., Wackher R.C., Linn C.B.: J. Chem. Phys. 44, 275 (1940)
Kul I., DesMarteau D.D., Beyerlein A.L.: Fluid Phase Equilib. 173, 263 (2000)
Peng D.Y., Robinson D.B.: Ind. Eng. Chem. Fundam. 15, 59 (1976)
Chueh C.F., Swanson A.C.: Chem. Eng. Prog. 69, 83 (1973)
Lee B.I., Kesler M.G.: AIChE J. 21, 510 (1975)
Lemmon E.W., McLinden M.O., Wagner W.: J. Chem. Eng. Data 54, 3141 (2009)
Wu J.T., Zhou Y., Lemmon E.W.: J. Phys. Chem. Ref. Data 40, 023104 (2011)
Span R., Wagner W.: Int. J. Thermophys. 18, 1415 (1997)
Smyth C.P., McAlpine K.B.: J. Chem. Phys. 2, 499 (1934)
Parthasarathy S.: Proc. Indian Natl. Sci. Acad. Sect. A 2, 497 (1935)
Grosse A.V.: J. Am. Chem. Soc. 59, 2739 (1937)
Vidaurri F.C.: J. Chem. Eng. Data 20, 349 (1975)
Chen Q., Hong R.H., Chen G.M.: J. Chem. Eng. Data 50, 1586 (2005)
Han X.H., Chen G.M., Li C.S., Qiao X.G., Cui X.L., Wang Q.: J. Chem. Eng. Data 51, 1232 (2006)
Dong X., Gong M., Zhang Y., Wu J.: J. Chem. Eng. Data 53, 2193 (2008)
Chen Q., Hong R.H., Chen G.M.: Fluid Phase Equilib. 237, 111 (2005)
Q. Chen, Ph.D. Dissertation, Zhejiang University, 2006
Bignell C.M., Dunlop P.J.: J. Chem. Phys. 98, 4889 (1993)
Stull D.R., Mayfield F.D.: Ind. Eng. Chem. 35, 639 (1943)
Smith D.C., Saunders R.A., Nielsen J.R., Ferguson E.E.: J. Chem. Phys. 20, 847 (1952)
Minkin D.M., Vvedenskii A.A.: Zh. Fiz. Khim. 41, 1571 (1967)
Chen S.S., Rodgers A.S., Chao J., Wilhoit R.C., Zwolinski B.J.: J. Phys. Chem. Ref. Data 4, 441 (1975)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, J., Zhou, Y. An Equation of State for Fluoroethane (R161). Int J Thermophys 33, 220–234 (2012). https://doi.org/10.1007/s10765-011-1151-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-011-1151-3