Abstract
With the ongoing global biodiversity crisis in the Anthropocene, it is critical to understand how to save endangered species to “bend the curve” of biodiversity decline. The Hainan gibbon (Nomascus hainanus) is a Critically Endangered species that is endemic to Hainan Island. We performed two synchronized total count surveys in Hainan Tropic Rain Forest National Park in November and December of 2020 and 2021 by locating gibbon groups from their morning calls and conducting detailed counts in all potential habitat fragments. We compared our findings with existing data to model the population trend, and analyzed the potential and realized reproductive potentials. We found 5 groups with a total of 33 gibbons in 2020 and 35 in 2021, including 4 and 6 solitary individuals respectively. This is an increase of 169% since 2003, when there were 13 individuals with 2 groups and 2 solitary individuals. Logistic and linear curves fitted the 2003-2021 population census data equally well. Although the population is growing, it has not realized its full reproductive potential (when all adult females give births at 24-month intervals), suggesting that external factors like available habitat, as well as nutritional, physiological, and behavioral factors may be limiting the population. The gibbon’s recovery demonstrates that establishing a nature reserve with regular patrols, banning logging, curbing poaching, and environmental education have been effective. Because the Hainan gibbon population is still extremely small, carefully planned conservation actions, including an ambitious forest restoration program, will be needed to ensure the species’ continued survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many primate species are threatened, and an understanding of their population status and factors that contribute to their threatened status is crucial for developing informed conservation strategies (Estrada et al., 2017). The evaluation of population and habitat conservation requires long-term field monitoring, such as the monitoring of the primate populations of Kibale National Park, Uganda (Chapman et al., 2010; Sarkar et al., 2021) and of kipunjis (Rungwecebus kipunji) in Tanzania (Davenport et al., 2022). However, such long-term field data often are lacking (Kleiman et al., 2001).
Fecundity is a significant parameter when evaluating a population (Bronikowski et al., 2011; Morris et al., 2011). The potential fecundity of a population is the maximum number of offspring born in the population when intrinsic and external factors are ideal, and all mature females breed (Bradshaw & McMahon, 2008). The potential fecundity may not be fully realized if fertilization is not successful despite mating or if females do not successfully bring a fetus to term due to nutritional, physiological, or behavioral factors.
The Hainan gibbon (Nomascus hainanus) inhabits the dense tropical forests of Hainan Island and lives in groups with a fixed home range (Liu & Qin, 1990; Bryant et al., 2016). Their habitat is fragmented by plantations, farmlands, villages, and roads in low altitude areas (Zhang et al., 2010; Zhang & Zang, 2018). Like other gibbons, adult group members give resonant morning songs and can be heard kilometers away (Bryant et al., 2017). When the weather is good, gibbons sing several times a day, but when the weather is rainy or cold, they sing less or stop (Deng et al., 2014; Zhou, 2008). Group composition is dynamic. Individuals leave groups when reaching sexual maturity and temporarily become solitary before joining other groups or finding mates to form new groups (Chan, Lo, & Mo, 2020a; Deng et al., 2017; Zhou, 2008).
Originally the Hainan gibbon likely inhabited much of Hainan Island (27,000 km2). However, hunting and habitat destruction caused population declines, especially between the 1950s and 1960s (Liu et al., 1984; Liu & Qin, 1990; Jiang et al., 1999; Turvey et al., 2015; Zhou et al., 2005). By the 1970s only a single population of fewer than ten gibbons survived in a single forest patch in the Bawangling area of Hainan Island (Liu et al., 1989).
To protect the gibbons, their remaining habitat was transformed from a state-run forest farm to Bawangling Provincial Nature Reserve (2,139 ha) in 1980, which was upgraded to Bawangling National Nature Reserve (BWLNNR) in 1988 (6,626 ha). BWLNNR encompassed the species’ entire known range, and was routinely patrolled by forest rangers. China implemented the Wild Animal Protection laws in 1989, and the Hainan gibbon was listed as a Class I protected species enjoying total protection, but BWLNNR did not prohibit commercial timber harvesting until the Hainan provincial government banned logging in 1994. In 2003, BWLNNR was expanded to 29,980 ha and became an integral part of the Hainan Tropical Rainforest National Park (HNTRNP) in 2019 (Zong, 2020).
To date, Hainan gibbon conservation has involved a series of essential steps. First, government leaders and conservation institutions appreciated the importance of Hainan gibbons, and their early adoption of emergency conservation measures such as reserve establishment was crucial. Second, the threats that lead to population declines were identified, and measures were implemented to reduce them. This included enforcing wildlife protection laws. Third, domestic and international conservation organizations played important roles by promoting community development and mobilizing local residents in gibbon monitoring and protection. In October 2021, the Chinese government announced the formal establishment of five national parks, including HNTRNP, demonstrating their commitment to protect rare and endangered species.
The Hainan gibbon population has grown gradually over the past few decades (Chan, Lo, & Mo, 2020a; Deng et al., 2017; Fellowes et al., 2008; Geissmann & Bleisch, 2020; Turvey et al., 2015; Wu et al., 2004). From 2003 to 2019, BWLNNR and the Hong Kong-based NGO Kadoorie Farm and Botanic Garden conducted six synchronized surveys using standardized survey methods (Chan, Lo, & Mo, 2020a; Fellowes et al., 2008). They found only two groups in 2003, but by 2015 there were four groups, and by 2019 there were five. Correspondingly, the population size increased from 13 gibbons in 2003 to 23 gibbons in 2013, and 32 individuals in 2019. The Hainan gibbon is the only Critically Endangered gibbon species whose population has grown (Geissmann & Bleisch, 2020).
We conducted synchronized field surveys in all known potential habitat patches of the Hainan gibbon in HNTRNP in 2020 and 2021. We used the same standardized methods as in the previous surveys, to update their population status, assess their potential and realized fecundity, and predict their population recovery under current management.
Methods
Study Area
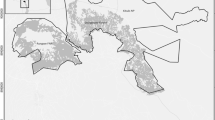
Since the 1980s, the Hainan gibbon has only been found in the mountainous Bawangling area in southwest Hainan Island (18°57'-19°11' N and 109°03'-109°17' E). BWLNNR covers 29,980 ha and ranges from 350 to 1560 m asl. The tropical monsoon climate has a rainy season from May to October and a dry season from November to April. The mean annual temperature is 21.3 °C and the annual rainfall is 1,657 mm. The vegetation varies with altitude and consists of the tropical deciduous seasonal rain forest, tropical evergreen seasonal rain forest, tropical mountain rain forest, tropical ravine rain forest, coniferous forest and shrub, and mountain top elfin forest. Since the 1980s, the gibbons have been closely monitored by researchers and rangers of the BWLNNR.
Synchronized Survey Protocols
Based on the methods of previous synchronized surveys (Fellowes et al., 2008) and our experiences of routine monitoring, we selected eight survey camp locations: Honghegu, Lingbagang, Shizilu, Miaocun, Kuiyegang, Laodian, Dongwuqu, and Dongbengling (Fig. 1). We set two or three fixed monitoring points around each camp based on the terrain and existing foot paths. We used a total of 20 fixed monitoring points, covering the area where Hainan gibbons were most likely to occur. Two or three investigators arrived at each monitoring point before. We recorded gibbon songs with a HUAWEI P30 cellphone and BOYA-MMI microphone, used a compass to determine their direction, and estimated the distance to the singing gibbons based on the volume of the song. This allowed us to triangulate the position of each group. Subsequently we searched for the gibbons and when we saw them, approached them as close as possible, determined the sex and age category of all individuals, and recorded their GPS location. We then contacted the other survey teams to ensure that the group was not double counted by two teams.
Study area. The map on the upper left is Hainan Rain Forest National Park (HNTRNP), and the map on the lower left is the study area inside HNTRNP. The vegetation map provides details of the study area with the home ranges of Hainan gibbons. The locations of gibbon groups are based on cumulative records from synchronized field surveys, patrols, and the archives of the Bawangling Sub-Management Bureau of HNTRNP 2017-2021.
As with previous surveys of the Hainan gibbon, our survey lasted 5 days, from November 12-17, 2020. After a 5-day repeated synchronized survey, we conducted a 3-day supplementary survey in Dongwuqu from November 18-20, 2020 to search for two solitary females seen in 2019, but not in the 2020 survey.
We performed another synchronized survey with the same survey protocol from November 29 to December 3, 2021, and conducted a 3-day supplementary survey for solitary gibbons in Caidi, experimenting with acoustic playback of gibbon singing to study the behavior of the solitary gibbons.
Population Parameter Calculation and Model Fitting
We compiled a dataset with records in the archive of BWLNNR and the literature. We used a linear equation and a logistic equation to fit the population data. We compared the goodness of fit of the two models using AIC to see if the logistic model describes the self-limiting growth of the Hainan gibbon better than the linear equation (Hilbe, 2009).
Mean Annual Growth Rate
We calculated the index of population annual growth rate from 2003 to 2021 using the following equation (Caughley, 1977):
Where: R is the annual growth rate; N0 is the initial population number; Nt is the population quantity in t year; t is the number of years.
Finite Rate of Growth
The formula for calculating the Finite Rate of Growth is:
Where: λ is the finite rate of growth; rm is the instantaneous growth rate. When λ > 1.00, the population increased. When λ = 1.00, the population is stable. When 0 < λ < 1.00, the population decreased. When λ = 0, the population will go extinct within one generation.
Instantaneous Growth Rate and Carrying Capacity
We fitted the survey data from 2003 to 2021 with a linear equation f(x) = a*x + b and a logistic curve equation f(x) = K/(1+e^(a-rt)) (Li et al., 2017), where a, b were constants to be estimated, and r was instantaneous growth rate.
We used the “Custom Equation” of the “Curve Fitting Tool” of MATLAB R2014a (Hahn & Valentine, 2019) and chose the learning algorithm based on the Levenberg Marquardt algorithm to fit the population census data and calculate the instantaneous growth rate, carrying capacity, and goodness of fit.
Potential Fecundity, Realized Fecundity, and Standardized Unrealized Reproductive Potential
Potential Fecundity (PF) is the total number of fertile females during the period divided by the interbirth interval (the interbirth interval of the Hainan gibbon is 24 months, Zhou et al., 2008) and Realized Fecundity (RF) is the number of babies born in the population in the year.
To standardize the ΔPF – RF which increases as population size increases, we propose a Standardized Un-realized Reproductive Potential (SURP) calculated based on the difference of PF and RF, and the Potential Fecundity, and expressed as a percentage:
Ethical Note
This study was performed in compliance with the relevant laws and regulations of the government of China and Hainan province on key protected wild animals and was approved by the Bureau of HNTRNP. The study only involved field observations and did not involve capturing or collaring gibbons. Field investigators were required to wear camouflage clothing and minimize noise during surveys, so the gibbons were disturbed as little as possible. This study was conducted in compliance with IPS Code of Best Practices for Field Primatology. The authors declare no competing financial interests.
Results
Population Status
The Hainan gibbon population numbered 33 in 2020 and 35 in 2021 (Tables I and II). The mean annual growth rate between 2003 and 2021 was 0.055, the instantaneous growth rate was 0.072, and the finite rate of growth was 1.075. Group size increased from 5.5 ± 0.5 (mean ± SD) in 2003 to 5.8 ± 1.7 in 2021. From 2019 to 2020, the group size of Group A remained at 6, Group B decreased from 8 to 7, Group C decreased from 10 to 8, Group D increased from 4 to 5, and Group E increased from 2 to 3. While only 2 solitary females were found in Dongwuqu in 2019, we spotted 4 solitary gibbons in 2020, including a solitary male and an immature individual of unidentified sex near Group A, and a solitary male and a solitary female close to Group B (Table II). During the 2021 synchronous survey, the group sizes were the same as in 2020, but we found 6 solitary gibbons, of which 4 were likely the same as the 4 solitary gibbons in 2020, and 2 new solitary females were spotted in Caidi, between the home ranges of Group B, Group C, and Group E (Fig. 2). These two solitary females in Caidi were found together, and during the supplementary survey they responded to the playing of a male song with a chorus typically given by groups.
Potential Fecundity vs. Realized Fecundity
Fecundity was not fully realized in the Hainan gibbon between 2017 to 2021 (Table III). The discrepancy (ΔPF - RF) between potential fecundity (PF) and realized fecundity (RF) of the gibbon population was 2.0 in 2017, narrowed to 0.5 in 2018-2019 and increased again in 2020-2021. Between 2017 and 2021, the potential fecundity was 18.5, but the realized fecundity was 10, the Standardized Unrealized Reproductive Potential of the Hainan gibbon population in 2017-2021 was only 46% (Table III). We did not analyze the reproductive potential before 2017 because too few births records were available.
Population Trajectory
The logistic model (SSE (Sum of Squared Error): 21.94; R-square: 0.9691; Adjusted R-square: 0.9665; AIC: 10.2864) and the linear model (SSE: 24.98: R-square: 0.9648; Adjusted R-square: 0.9618; AIC: 12.1055) fit the population trajectory of the Hainan gibbon from 2003 to 2021 equally well (Fig. 3).
Discussion
Our survey data confirm that the Hainan gibbon population has increased markedly over the past four decades, growing from 7-9 gibbons in the late 1980s to 35 gibbons in 2021. This confirms that the conservation measures implemented to protect the gibbons have been effective (Geissmann & Bleisch, 2020; Jiang et al., 2021).
The reproductive potential fluctuated in the Hainan gibbon population, but reproduction did not reach its maximum potential in the years we studied. Between 2017 and 2021, less than half of the potential fecundity was realized. Although female gibbons give birth at roughly 24-month intervals (Zhou et al., 2008), breeding may be limited by intrinsic factors such as sperm quality and the probability of fertilization in each menstrual cycle (Cords & Chowdhury, 2010; Mitani, 1990); stress during pregnancy, gestation, and parental care; possible aging of the breeding females (Caro et al., 1995; Nakagawa et al., 2003); and inbreeding owning to the very small initial population size (Guo et al., 2020). Extrinsic factors including food resources, nutrient content, patterns of food distribution, and possible human interference also can limit successful breeding (Hamilton, 1985). The roles of intrinsic and external factors in the fecundity of this gibbon population need further investigation. However, as intrinsic factors cannot be easily modified in a wild population, effort should be made to improve extrinsic limiting factors. This should involve the restoration of forest to create more habitats, which are abundant with nutritious food and free of disturbance for the gibbons, and construction of habitat corridors to promote dispersal and genetic diversity.
Since 2019, there have been five groups of Hainan gibbon—the highest number in the past 4 decades. Two groups were identified in 2003 (Chan et al., 2005) and the third, fourth, and fifth groups were formed in 2011, 2015, and 2019 respectively (Chan, Lo, & Mo, 2020a). New groups formed mainly in the original distribution area (Futouling area). Group E’s home range (Dongbenling area) is further from the original population but the newly established group consists of only three individuals (Chan, Lo, & Mo, 2020a). When there are fewer than five gibbons in a group, there is a high risk of group extinction (Turvey et al., 2015), so Group E should receive particular attention.
It is unclear why we never saw the two solitary females seen in Dongwuqu in 2019 again in our surveys. The two solitary females seen in Caidi in 2021 are not the same two gibbons seen in Dongwuqu in 2019, because the Dongququ females were already fully yellow in 2019, whereas the Caidi females were still half yellow and half black when the monitoring staff observed them early in March 2021. Solitary gibbons play an important role in group dynamics. On reaching sexual maturity, young gibbons are expelled by adult males and travel over a wider area without defined home ranges (Zhou, 2008). They usually live alone and do not have the typical and regular singing behaviors like mated adults in established groups (Deng et al., 2014). Leaving their natal group reduces the chances of inbreeding. Solitary gibbons may have higher mortality than group members, because they have no fixed home range and may have insufficient food, and some may be killed by hunters who mistake them for giant squirrels (Zhou, 2008).
The two solitary females in Caidi in 2021 were first discovered in March 2021. They were tracked and observed for 2 or 3 days by the monitoring staff each month, and they were always found together. It is reasonable to expect that if a male gibbon joins the females, they are likely to form a new group.
Although a new Hainan Gibbon group was detected using acoustic call playback (Bryant et al., 2017), acoustic playback was not the protocol we took for our synchronous surveys, and it was not used at all fixed listening points (including Caidi) during our synchronous surveys. We only experimentally used the sound playback to confirm their behavioral response during the supplementary survey in Caidi, where two solitary females have been found by investigators on December 2, 2021 during the synchronous surveys. The acoustic playback did not detect “additional animals” and did not influence the results. There is no temporal comparison involved here, so there is no potential for bias.
The conservation of group-living primates requires habitat patches that can support the entire group or connectivity among patches (Arroyo-Rodríguez et al., 2020; Chapman et al., 2007; Zhang et al., 2010). The number and area of habitat patches available for the Hainan gibbon are very limited (Turvey et al., 2015), and the quality of habitat patches varies (Zhang & Zang, 2018). Because Hainan gibbons have specific home range requirements (Bryant et al., 2017; Liu et al., 1989; Zhou et al., 2008), the existing habitat may become saturated as the population increases. Thus, improving habitat availability should be a priority, and a forest restoration project should be established (Chapman, 2018; Chapman et al., 2020).
Gibbon food is likely most abundant in natural old-growth lowland forests (Zhang & Zang, 2018). However, currently the gibbons in the Bawangling area are only found in botanically less diverse suboptimal habitat between 800 and 1,100 m (Guo et al., 2020). Although our knowledge of Hainan gibbon habitat requirements is limited, their habitat is fragmented, impeding dispersal. Above 1,200 m, trees of the Fagaceae Family are dominant, and these forests are not suitable for the gibbons, although they can still travel in this forest (Zhang & Zang, 2018). Lower altitudes have been developed into pine, rubber tree, or fruit tree plantations, farmlands, and villages, which are significant barriers to gibbon dispersal (Fig. 1). Restoring native forests at low altitudes will provide suitable habitat and facilitate gibbon dispersal.
The managers of HNRTNP and partners have been exploring habitat restoration measures for the Hainan gibbons. Forest restoration operations are planned for areas that are now pine, rubber tree, or fruit plantations. Gibbon food plants are being planted to enrich degraded forest patches. Tree species bearing fruits in the lean dry season have been targeted for restoration efforts and researchers are studying the feasibility of planting lianas with short fruiting periods and rich fruits. Other measures also are being implemented. For instance, an artificial rope bridge was set up in a degraded area to facilitate gibbon movement (Chan, Lo, Hong, et al., 2020b). Future research should address how to construct forest corridors to facilitate dispersal of the Hainan gibbon from its current range in the Futouling area to the well-forested Yajiadaling Mountains to the west (linear distance: 9-10 km) and to the Yinggeling Mountains in the east (linear distance: 35 km) (Fig. 1).
Conservation Significance
Because the Hainan gibbon population is extremely small, carefully planned solutions are needed to conserve them, and these in situ plans must include habitat restoration. Moreover, the insights gained from the holistic conservation approach used to conserve the Hainan gibbon can inform conservation plans for other primates. Our research highlights the importance of protecting mature rain forests, restoring habitat, and constructing habitat corridors. While detailed approaches vary by species and region, conservation actions to curb poaching, gain community support, establish protected areas, restore degraded habitat, and connect habitat patches are critical conservation measures that should be considered for many endangered primate species.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Arroyo-Rodríguez, V., Fahrig, L., Tabarelli, M., Watling, J. I., Tischendorf, L., Benchimol, M., Cazetta, E., Faria, D., Leal, I. R., & Melo, F. P. (2020). Designing optimal human-modified landscapes for forest biodiversity conservation. Ecology Letters, 23, 1404–1420.
Bradshaw, C. J. A. & McMahon, C. R.(2008). Fecundity. In Sven Erik Jørgensen and Brian D. Fath (Editor-in-Chief), Population Dynamics. Vol. 2 of Encyclopedia of Ecology. pp. 1535-1543. Elsevier.
Bronikowski, A. M., Altmann, J., Brockman, D. K., Cords, M., Fedigan, L. M., Pusey, A., Stoinski, T., Morris, W. F., Strier, K. B., & Alberts, S. C. (2011). Ageing in the natural world: comparative data reveal similar mortality patterns across primates. Science, 331, 1325–1328.
Bryant, J. V., Brulé, A., Wong, M. H. G., Hong, X., Zhou, Z., Han, W., Jeffree, T. E., & Turvey, S. T. (2016). Detection of a New Hainan Gibbon (Nomascus hainanus) Group Using Acoustic Call Playback. International Journal of Primatology, 37(4-5), 534–547.
Bryant, J. V., Zeng, X., Hong, X., Chatterjee, H. J., & Turvey, S. T. (2017). Spatiotemporal requirements of the Hainan gibbon (Nomascus hainanus): how much does home range constrain the recovery of the world’s rarest ape? American Journal of Primatology, 79(3), e22617. https://doi.org/10.1002/ajp.22617.
Caro, T. M., Sellen, D. W., Parish, A., Frank, R., Brown, D. M., Voland, E., & Muider, M. B. (1995). Termination of reproduction in nonhuman and human female primates. International Journal of Primatology, 16, 205–220.
Caughley, G. (1977). Analysis of Vertebrate Population. John Wiley & Sons.
Chan, B.P.L., Fellowes, J., Geissmann, T., & Zhang, J. (2005). Hainan gibbon Status Survey and Conservation Action Plan, Version 1 (last updated in November 2005). Kadoorie Farm and Botanic Garden Technical Report No. 3. Kadoorie Farm and Botanic Garden, Hong Kong.
Chan, B. P. L., Lo, Y., & Mo, Y. (2020a). New hope for the Hainan gibbon: formation of a new group outside its known range. Oryx, 54(3), 296–298.
Chan, B. P. L., Lo, Y. F. P., Hong, X. J., Mak, C. F., & Ma, Z. (2020b). First use of artificial canopy bridge by the world’s most critically endangered primate the Hainan gibbon Nomascus hainanus. Scientific Reports, 10, 15176. https://doi.org/10.1038/s41598-020-72641-z.
Chapman, C. A. (2018). A road for a promising future for China's primates: The potential for restoration. Zoology Research, 39, 244–248.
Chapman, C. A., Naughton-Treves, L., Lawes, M. J., Wasserman, M. D., & Gillespie, T. R. (2007). The conservation value of forest fragments: Explanations for population declines of the colobus of western Uganda. International Journal of Primatology, 23, 513–578.
Chapman, C. A., Struhsaker, T. T., Skorupa, J. P., Snaith, T. V., & Rothman, J. M. (2010). Understanding long-term primate community dynamics: implications of forest change. Ecological Applications, 20, 179–191.
Chapman, C. A., Bicca-Marques, J. C., Dunham, A. E., Fan, P., Fashing, P. J., Gogarten, J. F., Guo, S., Huffman, M. A., Kalbitzer, U., Li, B., Ma, C., Matsuda, I., Omeja, P. A., Sarkar, D., Sengupta, R., Serio-Silva, J. C., Tsuji, Y., & Stenseth, N. C. (2020). Primates can be a rallying symbol to promote tropical forest restoration. Folia Primatologica, 91, 669–687.
Cords, M., & Chowdhury, S. (2010). Life History of Cercopithecus mitis stuhlmanni in the Kakamega Forest, Kenya. International Journal of Primatology, 31, 433–455.
Davenport, T. R. B., Machaga, S. J., Mpunga, N. E., Kimiti, S. P., Mwalwengele, W., Mwaipungu, O., & Makumbule, P. M. (2022). A reassessment of the population size, demography, and status of Tanzania’s endemic Kipunji Rungwecebus kipunji 13 years on: Demonstrating conservation success. International Journal of Primatology. https://doi.org/10.1007/s10764-022-00281-3.
Deng, H., Zhou, J., & Yang, Y. (2014). Sound spectrum characteristics of songs of Hainan gibbon (Nomascus hainanus). International Journal of Primatology, 35(2), 547–556.
Deng, H., Zhang, M., & Zhou, J. (2017). Recovery of the critically endangered Hainan gibbon Nomascus hainanus. Oryx, 51(1), 161–165.
Estrada, A., Garber, P. A., Rylands, A. B., Roos, C., Fernandez-Duque, E., Fiore, A. D., Nekaris, K. A.-I., Nijman, V., Heymann, E. W., Lambert, J. E., Rovero, F., Barelli, C., Setchell, J. M., Gillespie, T. R., Mittermeier, R. A., Arregoitia, L. V., de Guinea, M., Gouveia, S., Dobrovolski, R., et al (2017). Impending extinction crisis of the world’s primates: Why primates matter. Science Advances, 3(1), e1600946.
Fellowes, J. R., Chan, B. P. L., Zhou, J., Chen, S., Yang, S., & Ng, S. C. (2008). Current status of the Hainan gibbon (Nomascus hainanus): progress of population monitoring and other priority actions. Asian Primates Journal, 1, 2–9.
Geissmann, T., & Bleisch, W. (2020). Nomascus hainanus. The IUCN Red List of Threatened Species 2020: e.T41643A17969392. https://doi.org/10.2305/IUCN.UK.2020-2.RLTS.T41643A17969392.en
Guo, Y., Chang, J., Han, L., Liu, T., Li, G., Garber, P. A., Xiao, N., & Zhou, J. (2020). The genetic status of the Critically Endangered Hainan gibbon (Nomascus hainanus): A species moving toward extinction. Frontiers in Genetics. https://doi.org/10.3389/fgene.2020.608633.
Hahn, B. D., & Valentine, D. T. (2019). Essential MATLAB for Engineers and Scientists. Academic Press.
Hamilton, M. J. (1985). Demographic Consequences of a Food and Water Shortage to Desert Chacma Baboons, Papio ursinus. International Journal of Primatology, 6(5), 451–462.
Hilbe, J. M. (2009). Logistic Regression Models. CRC.
Jiang, H.S., Song, X., & Zhang, J.(1999) The population dynamic of Hylobates concolor hainanus in Bawangling National Nature Reserve in Hainan Island (Unpublished report).
Jiang, Z., Wu, Y., Liu, S., Jiang, X., Zhou, K., & Hu, H. (2021). China’s Red list of Biodiversity: Vertebrates, Volume I, Mammals. Science Press.
Kleiman, D. G., Reading, R. P., Miller, B. J., Clark, T. W., Scott, J. M., Robinson, J., Wallace, R. L., Cabin, R. J., & Fellman, F. (2001). Improving the evaluation of conservation programs. Conservation Biology, 14(2), 356–365.
Li, H., Bai, Y., & Li, Z. (2017). An improved parameter estimation method of logistic model and its application. Mathematics in Practice and Theory, 22, 185–190.
Liu, Z., & Qin, C. (1990). Analysis of Hainan gibbon habitat structure. Acta Theriologica Sinica, 10(3), 163–169.
Liu, Z., Yu, S., & Yuan, X. (1984). Resource status of Hainan gibbon. Chinese Journal of Wildlife, 6(6), 1–4.
Liu, Z., Zhang, Y., Jiang, H., & Southwick, C. (1989). Population structure of Hylobates concolor in Bawanglin Nature Reserve, Hainan, China. American Journal of Primatology, 19, 247–254.
Mitani, J. C. (1990). Demography of agile gibbons (Hylobates agilis). International Journal of Primatology, 11, 411–424.
Morris, W. F., Altmann, J., Brockman, D. K., Cords, M., Fedigan, L. M., Pusey, A. E., Stoinski, T. S., Bronikowski, A. M., Alberts, S. C., & Strier, K. S. (2011). Low demographic variability in wild primate populations: fitness impacts of variation, covariation, and serial correlation in vital rates. The American Naturalist, 177, 14–28.
Nakagawa, N., Ohsawa, H., & Muroyama, Y. (2003). Life-history parameters of a wild group of West African patas monkeys (Erythrocebus patas patas). Primates, 44(3), 281–290.
Sarkar, D., Bortolamiol, S., Gogarten, J. F., Hartter, J., Hou, R., Kagoro, W., MacKenzie, C., Omeja, P., Reyna-Hurtado, R., Tumwesigye, C., & Chapman, C. A. (2021). Exploring multiple dimensions of conservation success: Long-term wildlife trends, anti-poaching efforts, and revenue sharing in Kibale National Park. Animal Conservation. https://doi.org/10.1111/acv.12765.
Song, X., Jiang, H., Zhang J., Chen, Q., Wang, C., & Lin, W. (1999). Population survey of black gibbon in Hainan. Animal Science in China - Proceedings of the 14th Congress and the 65th annual meeting of the Chinese Society of Zoology: 696-701.
Turvey, S. T., Traylor-Holzer, K., Wong, M. H. G., Bryant, J. V., Zeng, X., Hong, X., & Long, Y. (2015). International Conservation Planning Workshop for the Hainan Gibbon: Final Report. Zoological Society of London/IUCN SSC Conservation Breeding Specialist Group.
Wu, W., Wang, X. M., Claro, F., Ding, Y. Z., Souris, A. C., Wang, C. D., Wang, C. H., & Berzins, R. (2004). The current status of the Hainan black-crested gibbon Nomascus sp. cf. nasutus hainanus in Bawangling National Nature Reserve, Hainan, China. Oryx, 38(4), 452–456.
Zhang, Z., & Zang, R. (2018). Diversity and distribution of food plants: Implications for conservation of the critically endangered Hainan gibbon. Nature Conservation, 31, 17–33.
Zhang, M., Fellowes, J. R., Jiang, X., Wang, W., Chan, B. P. L., Ren, G., & Zhu, J. (2010). Degradation of tropical forest in Hainan, China, 1991–2008: Conservation implications for Hainan Gibbon (Nomascus hainanus). Biological Conservation, 143, 1397–1404.
Zhou, J. (2008). The ecology and behavior traits of Hainan black-crested gibbon (Nomascus hainanus). Unpublished Ph.D. dissertation, Northeast Normal University, Jilin, China.
Zhou, J., Wei, F., Li, M., Zhang, J., Wang, D., & Pan, R. (2005). Hainan black-crested gibbon is headed for extinction. International Journal of Primatology, 26, 453–465.
Zhou, J., Wei, F., Li, M., Chan, B. P. L., & Wang, D. (2008). Reproductive characters and mating behaviour of wild Nomascus hainanus. International Journal of Primatology, 29, 1037–1046.
Zong, L. (2020). The path to effective national park conservation and management: Hainan Tropical Rainforest National Park System Pilot Area. International Journal of Geoheritage and Parks, 8(4), 225–229.
Acknowledgments
This project is supported by a key project of the Hainan Provincial Bureau of Science and Technology (No. ZDYF2020179), the Strategic Leading Science and Technologic Project of the Chinese Academy of Sciences (Type A, No. XDA19050204). We acknowledge the leadership of the Zhang Xingsheng, President of the Council of Hainan Institute of National Park, we also thank those who participated or gave us their support during the study: Tang Yanfei, Fan Pengfei, Jin Kun, Shi Kun, Song Xiqiang, Liu Hui, Bao Heng, Du Yanjun, Yu Chenxing, Tu Feiyun, Deng Huaiqing, Zhong Xukai, Zhu Changyue, Feng Yuan, Li Ping, Ma Ziyu, Chen Yukai, Zhao Xingfeng, Song Qinchuan, Hu Shiqi, BI Zhiyong, Zhou Jinghe, CAI Ying, Wang Jiangang, Wang Wenqing, Lin Chong, Feng Yewang, Yang Donghua, Han Wentao, Zou Zhengchong, Chen Qing, Wang Jinqiang, Xie Zengnan, Huang Lubiao, Li Wenyong, Zhasng Zhicheng, Li Quanjin Lin Qing, Liu Huiqin, Xu Xiangdong, Tao Jinlin, and other members and rangers of the monitoring team of Bawangling Administration Sub-Management Bureau of HNRFNK. CAC was funded by the Wilson Center and BPLC was funded by Kadoorie Farm and Botanic Garden during the writing phase of this project. The authors thank Qingqing He and Shasha Yan for drawing the Fig. 1. The authors are grateful to the Handling Editor: Professor Joanna Setchell, the Editor-in Chief, and two anonymous reviewers for their help and comments on the manuscript.
Author information
Authors and Affiliations
Contributions
LG, JZ, and LX designing, coordinating, participating the field surveys, and writing the manuscript. XZ, QX, and ZJ, coordinating, participating the field surveys, LZ. CAC, BPLC, HX, and MY were involved in the analysis and writing the manuscript.
Corresponding author
Additional information
Handling Editor: Joanna Setchell
Inclusion and diversity statement
The author list includes contributors from the location where the research was conducted, who participated in study conception, study design, data collection, analysis, and interpretation of the findings.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, G., Lu, X., Liu, Z. et al. The Critically Endangered Hainan Gibbon (Nomascus hainanus) Population Increases but not at the Maximum Possible Rate. Int J Primatol 43, 932–945 (2022). https://doi.org/10.1007/s10764-022-00309-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-022-00309-8