Abstract
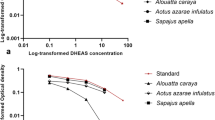
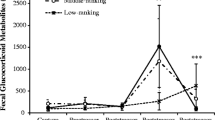
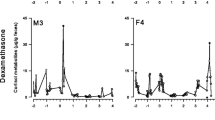
Measuring hormonal profiles is important in monitoring stress, physical fitness, and reproductive status in primates. Noninvasive methods have been used to measure several steroid hormones in primates without causing them stress. However, few studies have used feces or urine to measure dehydroepiandrosterone sulfate (DHEAS), an important precursor of sex steroids that has been studied as a biomarker of aging, pregnancy, and stress in humans and nonhuman primates. We developed an enzyme immunoassay to detect DHEAS in the feces of Japanese macaques (Macaca fuscata). Our subjects included eight singly housed Japanese macaques. To validate the assay, we administrated oral DHEA to one male and one female macaque, collected their feces, and measured DHEAS levels over time. Given that DHEAS is related to gonadal steroids and the stress response, we also measured DHEAS concentrations in response to adrenal (adrenocorticotropic hormone [ACTH]) and gonadal (human chorionic gonadotropin [hCG]) stimulation. Our assay successfully detected DHEAS in Japanese macaque feces, and levels of DHEAS were associated with the amount of DHEA ingested. Parallelism and accuracy tests revealed that fecal extracts were reliable measures of DHEAS. Neither ACTH nor hCG challenge appeared to affect DHEAS levels. The method we describe is less expensive than that using the commercially available kits and is applicable to investigations involving aging, stress, and reproduction in Japanese macaques.




Similar content being viewed by others
References
Alesci S, Bornstein S. (2001). Intraadrenal mechanisms of DHEA regulation: A hypothesis for adrenopause. Experimental and Clinical Endocrinology & Diabetes 109(2): 75–82.
Auchus, R. J., & Rainey, W. E. (2004). Adrenarche: Physiology, biochemistry and human disease. Clinical Endocrinology, 60(3), 288–296.
Baulieu, E. E. (1996). Dehydroepiandrosterone (DHEA): A fountain of youth? Journal of Clinical Endocrinology and Metabolism, 81(9), 3147–3151.
Baulieu, E. E., Thomas, G., Legrain, S., Lahlou, N., Roger, M., Debuire, B., Faucounau, V., Girard, L., Hervy, M. P., Latour, F., Leaud, M. C., Mokrane, A., Pitti-Ferrandi, H., Trivalle, C., de Lacharriere, O., Nouveau, S., Rakoto-Arison, B., Souberbielle, J. C., Raison, J., le Bouc, Y., Raynaud, A., Girerd, X., & Forette, F. (2000). Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: Contribution of the DHEAge study to a sociobiomedical issue. Proceedings of the National Academy of Sciences of the USA, 97(8), 4279–4284.
Behringer, V., & Deschner, T. (2017). Non-invasive monitoring of physiological markers in primates. Hormones and Behavior, 91, 3–18.
Behringer, V., Hohmann, G., Stevens, J. M., Weltring, A., & Deschner, T. (2012). Adrenarche in bonobos (Pan paniscus): Evidence from ontogenetic changes in urinary dehydroepiandrosterone-sulfate levels. Journal of Endocrinology, 214(1), 55–65.
Belanger, A., Candas, B., Dupont, A., Cusan, L., Diamond, P., et al (1994). Changes in serum concentrations of conjugated and unconjugated steroids in 40-year-old to 80-year-old men. The Journal of Clinical Endocrinology and Metabolism, 79(4), 1086–1090.
Bernstein, R. M., Sterner, K. N., & Wildman, D. E. (2012). Adrenal androgen production in catarrhine primates and the evolution of adrenarche. American Journal of Physical Anthropology, 147(3), 389–400.
Boudarene, M., Legros, J. J., & Timsit-Berthier, M. (2002). Study of the stress response: Role of anxiety, cortisol and DHEAS. Encephale, 28(2), 139–146.
Burger, H. G. (2002). Androgen production in women. Fertility and Sterility, 77(Suppl 4), S3–S5.
Burkhardt, T., Schmidt, N. O., Vettorazzi, E., Aberle, J., Mengel, M., & Flitsch, J. (2013). DHEA(S): A novel marker in Cushing's disease. Acta Neurochirurgica (Wien), 155(3), 479–484 discussion 484.
Campbell, B. (2011). Adrenarche in comparative perspective. American Journal of Human Biology, 23(1), 44–52.
Castracane, V. D., Cutler Jr., G. B., & Loriaux, D. L. (1981). Pubertal endocrinology of the baboon: Adrenarche. American Journal of Physiology, 241(4), E305–E309.
Copeland, K. C., Eichberg, J. W., Parker Jr., C. R., & Bartke, A. (1985). Puberty in the chimpanzee: Somatomedin-C and its relationship to somatic growth and steroid hormone concentrations. Journal of Clinical Endocrinology and Metabolism, 60(6), 1154–1160.
Cutler Jr., G. B., Glenn, M., Bush, M., Hodgen, G. D., Graham, C. E., & Loriaux, D. L. (1978). Adrenarche: A survey of rodents, domestic animals, and primates. Endocrinology, 103(6), 2112–2118.
Degen, L. P., & Phillips, S. F. (1996). Variability of gastrointestinal transit in healthy women and men. Gut, 39(2), 299–305.
Du, C. L., Lin, M. C., Lu, L., & Tai, J. J. (2011). Correlation of occupational stress index with 24-hour urine cortisol and serum DHEA sulfate among city bus drivers: A cross-sectional study. Safety and Health at Work, 2(2), 169–175.
Fehér, T., Szalay, K. S., & Szilágyi, G. (1985). Effect of ACTH and prolactin on dehydroepiandrosterone, its sulfate ester and cortisol production by normal and tumorous human adrenocortical cells. Journal of Steroid Biochemistry, 23(2), 153–157.
Flood, J. F., & Roberts, E. (1988). Dehydroepiandrosterone sulfate improves memory in aging mice. Brain Research, 448(1), 178–181.
Goncharova, N. D., Vengerin, A. A., & Chigarova, O. A. (2012). Repeated moderate stress stimulates the production of dehydroepiandrosterone sulfate (DHEAS) and reduces corticosteroid imbalance in old Macaca mulatta. Bulletin of Experimental Biology and Medicine, 153(5), 750–753.
Hammer, F., Subtil, S., Lux, P., Maser-Gluth, C., Stewart, P. M., Allolio, B., & Arlt, W. (2005). No evidence for hepatic conversion of dehydroepiandrosterone (DHEA) sulfate to DHEA: In vivo and in vitro studies. The Journal of Clinical Endocrinology & Metabolism, 90(6), 3600–3605.
Havelock, J. C., Auchus, R. J., & Rainey, W. E. (2004). The rise in adrenal androgen biosynthesis: Adrenarche. Seminars in Reproductive Medicine, 22(4), 337–347.
Hechter, O., Grossman, A., & Chatterton Jr., R. T. (1997). Relationship of dehydroepiandrosterone and cortisol in disease. Medical Hypotheses, 49(1), 85–91.
Heistermann, M., Palme, R., & Ganswindt, A. (2006). Comparison of different enzymeimmunoassays for assessment of adrenocortical activity in primates based on fecal analysis. American Journal of Primatology, 68(3), 257–273.
Herrera-Justiniano, E., Galvez, M., Aznar, A., Gomez, S., Sendon, P., Zurita, A. R., Malagon, C. M., & Aznar, R. A. (1979). Changes in the plasma levels of androstenedione, dehydroepiandrosterone and cortisol after stimulation with ACTH and hCG and suppression with dexamethasone during male puberty. Acta Endocrinologica, 90(1), 113–121.
Hornsby, P. J. (1995). Biosynthesis of DHEAS by the human adrenal cortex and its age-related decline. Annals of the New York Academy of Sciences, 774, 29–46.
Hu, Y., Cardounel, A., Gursoy, E., Anderson, P., & Kalimi, M. (2000). Anti-stress effects of dehydroepiandrosterone: Protection of rats against repeated immobilization stress-induced weight loss, glucocorticoid receptor production, and lipid peroxidation. Biochemical Pharmacology, 59(7), 753–762.
Kalmijn, S., Launer, L. J., Stolk, R. P., de Jong, F. H., Pols, H. A., et al (1998). A prospective study on cortisol, dehydroepiandrosterone sulfate, and cognitive function in the elderly. The Journal of Clinical Endocrinology and Metabolism, 83(10), 3487–3492.
Kemnitz, J. W., Roecker, E. B., Haffa, A. L., Pinheiro, J., Kurzman, I., et al (2000). Serum dehydroepiandrosterone sulfate concentrations across the life span of laboratory-housed rhesus monkeys. Journal Medical of Primatology, 29(5), 330–337.
Kroboth, P. D., Salek, F. S., Pittenger, A. L., Fabian, T. J., & Frye, R. F. (1999). DHEA and DHEA-S: A review. Journal of Clinical Pharmacology, 39(4), 327–348.
Laatikainen, T., Laitinen, E., & Vihko, R. (1971). Secretion of free and sulfate-conjugated neutral steroids by the human testis: Effect of administration of human chorionic gonadotropin. The Journal of Clinical Endocrinology & Metabolism, 32(1), 59–64.
Labrie, F. (2010). DHEA, important source of sex steroids in men and even more in women. Progress in Brain Research, 182, 97–148.
Labrie, F., Bélanger, A., Cusan, L., Gomez, J.-L., & Candas, B. (1997). Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. The Journal of Clinical Endocrinology and Metabolism, 82(8), 2396–2402.
Labrie, F., Luu-The, V., Belanger, A., Lin, S. X., Simard, J., et al (2005). Is dehydroepiandrosterone a hormone? The Journal of Endocrinology, 187(2), 169–196.
Lampe, J. W., Fredstrom, S. B., Slavin, J. L., & Potter, J. D. (1993). Sex differences in colonic function: A randomised trial. Gut, 34(4), 531–536.
Laughlin, G. A., & Barrett-Connor, E. (2000). Sexual dimorphism in the influence of advanced aging on adrenal hormone levels: The rancho Bernardo study. The Journal of Clinical Endocrinology and Metabolism, 85(10), 3561–3568.
Leblanc, M., Labrie, C., Belanger, A., Candas, B., & Labrie, F. (2002). Pharmacokinetics of oral dehydroepiandrosterone (DHEA) in the ovariectomised cynomolgus monkey. The Journal of Steroid Biochemistry and Molecular Biology, 81(2), 159–164.
Liu, T. C., Lin, C. H., Huang, C. Y., Ivy, J. L., & Kuo, C. H. (2013). Effect of acute DHEA administration on free testosterone in middle-aged and young men following high-intensity interval training. European Journal of Applied Physiology, 113(7), 1783–1792.
Majewska, M. D. (1995). Neuronal actions of dehydroepiandrosterone: Possible roles in brain development, aging, memory, and affect. Annals of the New York Academy of Sciences, 774, 111–120.
Maninger, N., Capitanio, J. P., Mason, W. A., Ruys, J. D., & Mendoza, S. P. (2010). Acute and chronic stress increase DHEAS concentrations in rhesus monkeys. Psychoneuroendocrinology, 35(7), 1055–1062.
McCraty, R., Barrios-Choplin, B., Rozman, D., Atkinson, M., & Watkins, A. D. (1998). The impact of a new emotional self-management program on stress, emotions, heart rate variability, DHEA and cortisol. Integrative Physiological and Behavioral Science, 33(2), 151–170.
Mesiano, S., & Jaffe, R. B. (1997). Developmental and functional biology of the primate fetal adrenal cortex. Endocrine Reviews, 18(3), 378–403.
Meusy-Dessolle, N., & Dang, D. C. (1985). Plasma concentrations of testosterone, dihydrotestosterone, delta 4-androstenedione, dehydroepiandrosterone and oestradiol-17 beta in the crab-eating monkey (Macaca fascicularis) from birth to adulthood. Journal of Reproduction and Fertility, 74(2), 347–359.
Mobbs, C. V. (1998). Dehydroepiandrosterone and aging. In C. V. Mobbs, P. R. Hof (Eds.), Functional endocrinology of aging (Vol. 29, pp. 217–227). Basel: Karger Publishers.
Möhle, U., Heistermann, M., Palme, R., & Hodges, J. K. (2002). Characterization of urinary and fecal metabolites of testosterone and their measurement for assessing gonadal endocrine function in male nonhuman primates. General and Comparative Endocrinology, 129(3), 135–145.
Monfort, S. L. (2003). Non-invasive endocrine measures of reproduction and stress in wild populations. In W. Holt, A. Pickard, J. Rodger, D. Wildt (Eds.), Reproductive science and integrated conservation conservation biology (Vol. 8, pp. 147–165). Cambridge: Cambridge University Press.
Moran, F. M., Chen, J., Gee, N. A., Lohstroh, P. N., & Lasley, B. L. (2013). Dehydroepiandrosterone sulfate levels reflect endogenous luteinizing hormone production and response to human chorionic gonadotropin challenge in older female macaque (Macaca fascicularis). Menopause, 20(3), 329–335.
Muehlenbein, M. P., Campbell, B. C., Richards, R. J., Svec, F., Phillippi-Falkenstein, K. M., Murchison, M. A., & Myers, L. (2003). Dehydroepiandrosterone-sulfate as a biomarker of senescence in male non-human primates. Experimental Gerontology, 38(10), 1077–1085.
Neunzig, J., & Bernhardt, R. (2014). Dehydroepiandrosterone sulfate (DHEAS) stimulates the first step in the biosynthesis of steroid hormones. PLoS One, 9(2), e89727.
Nieschlag, E., Loriaux, D. L., Ruder, H., Zucker, I., Kirschner, M., & Lipsett, M. (1973). The secretion of dehydroepiandrosterone and dehydroepiandrosterone sulphate in man. Journal of Endocrinology, 57(1), 123–134.
Orentreich, N., Brind, J. L., Rizer, R. L., & Vogelman, J. H. (1984). Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. The Journal of Clinical Endocrinology & Metabolism, 59(3), 551–555.
Palme, R. (2005). Measuring fecal steroids: Guidelines for practical application. Annals of the New York Academy of Sciences, 1046, 75–80.
Prall, S. P., Ambu, L., Nathan, S., Alsisto, S., Ramirez, D., & Muehlenbein, M. P. (2015). Androgens and innate immunity in rehabilitated semi-captive orangutans (Pongo pygmaeus morio) from Malaysian Borneo. American Journal of Primatology, 77(6), 642–650.
Rainey, W. E., Rehman, K. S., & Carr, B. R. (2004). The human fetal adrenal: Making adrenal androgens for placental estrogens. Seminars in Reproductive Medicine, 22(4), 327–336.
Remer, T., Manz, F., & Pietrzik, K. (1995). Re-examination of the effect of hCG on plasma levels and renal excretion of dehydroepiandrosterone sulfate in healthy males. Steroids, 60(2), 204–209.
Saez, J. M., & Bertrand, J. (1968). Studies on testicular function in children: Plasma concentrations of testosterone, dehydroepiandrosterone and its sulfate before and after stimulation with human chorionic gonadotrophin (1). Steroids, 12(6), 749–761.
Scott, L. V., Svec, F., & Dinan, T. (2000). A preliminary study of dehydroepiandrosterone response to low-dose ACTH in chronic fatigue syndrome and in healthy subjects. Psychiatry Research, 97(1), 21–28.
Seraphin, S. B., Whitten, P. L., & Reynolds, V. (2008). The influence of age on fecal steroid hormone levels in male Budongo Forest chimpanzees (Pan troglodytes schweinfurthii). American Journal of Primatology, 70(7), 661–669.
Siegel, S. F., Finegold, D. N., Lanesx, R., & Lee, P. A. (1990). ACTH stimulation tests and plasma dehydroepiandrosterone sulfate levels in women with hirsutism. The New England Journal of Medicine, 323(13), 849–854.
Sorwell, K. G., & Urbanski, H. F. (2010). Dehydroepiandrosterone and age-related cognitive decline. Age (Dordrecht, Netherlands), 32(1), 61–67.
Spivak, B., Maayan, R., Kotler, M., Mester, R., Gil-Ad, I., et al (2000). Elevated circulatory level of GABA(a): |antagonistic neurosteroids in patients with combat-related post-traumatic stress disorder. Psychological Medicine, 30(5), 1227–1231.
Takeshita, R. S., Huffman, M. A., Bercovitch, F. B., Mouri, K., & Shimizu, K. (2013). The influence of age and season on fecal dehydroepiandrosterone-sulfate (DHEAS) concentrations in Japanese macaques (Macaca fuscata). General and Comparative Endocrinology, 191C, 39–43.
Takeshita, R. S., Huffman, M. A., Mouri, K., Shimizu, K., & Bercovitch, F. B. (2016). Dead or alive? Predicting fetal loss in Japanese macaques (Macaca fuscata) by fecal metabolites. Animal Reproduction Science, 175, 33–38.
Takeshita, R. S. C., Bercovitch, F. B., Huffman, M. A., Mouri, K., Garcia, C., Rigaill, L., & Shimizu, K. (2014). Environmental, biological, and social factors influencing fecal adrenal steroid concentrations in female Japanese macaques (Macaca fuscata). American Journal of Primatology, 76(11), 1084–1093.
Takeshita, R. S. C., Huffman, M. A., Kinoshita, K., & Bercovitch, F. B. (2017). Effect of castration on social behavior and hormones in male Japanese macaques (Macaca fuscata). Physiology and Behavior, 181, 43–50.
Takeshita RSC, Bercovitch FB, Kinoshita K, Huffman MA (2018). Beneficial effect of hot spring bathing on stress levels in Japanese macaques. Primates. https://doi.org/10.1007/s10329-018-0655-x.
Touma, C., & Palme, R. (2005). Measuring fecal glucocorticoid metabolites in mammals and birds: The importance of validation. Annals of the New York Academy of Sciences, 1046(1), 54–74.
Touma, C., Sachser, N., Mostl, E., & Palme, R. (2003). Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. General and Comparative Endocrinology, 130(3), 267–278.
Vallee, M., Mayo, W., & Le Moal, M. (2001). Role of pregnenolone, dehydroepiandrosterone and their sulfate esters on learning and memory in cognitive aging. Brain Research Reviews, 37(1–3), 301–312.
Walsh, S. W., Stanczyk, F. Z., & Novy, M. J. (1984). Daily hormonal changes in the maternal, fetal, and amniotic-fluid compartments before parturition in a primate species. The Journal of Clinical Endocrinology and Metabolism, 58(4), 629–639.
Weinstein, R., Kelch, R., Jenner, M., Kaplan, S., & Grumbach, M. (1974). Secretion of unconjugated androgens and estrogens by the normal and abnormal human testis before and after human chorionic gonadotropin. Journal of Clinical Investigation, 53(1), 1–6.
Whitten, P. L., Brockman, D. K., & Stavisky, R. C. (1998). Recent advances in noninvasive techniques to monitor hormone-behavior interactions. American Journal of Physical Anthropology, 107(Suppl 27), 1–23.
Willis, E. L., Wolf, R. F., White, G. L., & McFarlane, D. (2014). Age- and gender-associated changes in the concentrations of serum TGF-1 beta, DHEA-S and IGF-1 in healthy captive baboons (Papio hamadryas anubis). General and Comparative Endocrinology, 195, 21–27.
Yamaji, T., & Ibayashi, H. (1969). Plasma dehydroepiandrosterone sulfate in normal and pathological conditions. The Journal of Clinical Endocrinology & Metabolism, 29(2), 273–278.
Zhou, R., Bird, I. M., Dumesic, D. A., & Abbott, D. H. (2005). Adrenal hyperandrogenism is induced by fetal androgen excess in a rhesus monkey model of polycystic ovary syndrome. The Journal of Clinical Endocrinology & Metabolism, 90(12), 6630–6637.
Acknowledgements
We thank Mr. Akihisa Kaneko, Ms. Mayumi Morimoto, and all support staff from the Center for Human Evolution Modeling Research at the Primate Research Institute for providing useful information and assistance during the experimental procedures. We would like to express our gratitude to the journal editor and reviewers for the English language suggestions and for the very useful comments to improve this manuscript. The study was funded by the Primate Research Institute, the Leading Program in Primatology and Wildlife Science (PWS), a grant-in-aid from the Japan Society for the Promotion of Science (JSPS) no. 16 J00399, and a scholarship to R. S. C. Takeshita by the Nippon Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Handling Editor: Joanna M. Setchell
Rights and permissions
About this article
Cite this article
Takeshita, R.S.C., Bercovitch, F.B., Huffman, M.A. et al. Development and Validation of an Enzyme Immunoassay for Fecal Dehydroepiandrosterone Sulfate in Japanese Macaques (Macaca fuscata). Int J Primatol 39, 208–221 (2018). https://doi.org/10.1007/s10764-018-0026-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-018-0026-x