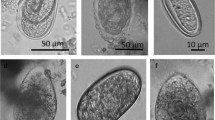
Abstract
Understanding pathogen transmission is essential to addressing the dynamics of infectious diseases in animal populations. Directly transmitted parasites spread in host populations via 1) contact with infected individuals and 2) contact with contaminated substrates. Although studies exist that support social or ranging effects on transmission, it is less clear how these factors interact. We test the hypothesis that a combination of social, ranging, diet, and intrinsic factors account for Trypanoxyuris minutus (pinworm) infections in sympatric howler species Alouatta palliata and A. pigra. We collected 211 howler fecal samples from 34 adults living in four groups, two of each species, in Tabasco (Mexico), and calculated pinworm prevalence and eggs per gram of feces (EPG). We followed each group for 80 h to determine ranging, diet, frequency of contact, and conspecific proximity. Prevalence of Trypanoxyuris minutus was high, with 82% of all individuals infected. Logistic modeling indicated that pinworm prevalence was positively associated with proximity and the proportion of group members contacted by focal individuals. Although EPG results should be interpreted cautiously owing to variable egg excretion, this index was also positively associated with proximity and the proportion of group members that were contacted, as well as with dietary diversity and use of non-tree foods. Neither intrinsic factors such as species and sex, nor group and population level variables, such as group and home range size, home range overlap, and intensity of range use, were significant predictors of pinworm infection. We conclude that both sociality and feeding behavior are key factors in infection dynamics of Trypanoxyuris minutus in sympatric Alouatta palliata and A. pigra, confirming that contact with infected conspecifics and contaminated substrates are important mechanisms for directly transmitted parasites.


Similar content being viewed by others
References
Adamson, M. (1994). Evolutionary patterns in the life histories of Oxyurida. International Journal of Parasitology, 24, 1167–1177.
Aldeen, W. E., Shisenant, J., Hale, D., Matsen, J., & Carroll, K. (1993). Comparison of pooled formalin-preserved fecal specimens with three individuals samples for detection of intestinal parasites. Journal of Clinical Microbiology, 31, 144–145.
Altizer, S., Nunn, C. L., Thrall, P. H., Gittleman, J. L., Antonovics, J., Cunningham, A. A., Dobson, A. P., Ezenwa, V., Jones, K. E., Pedersen, A. B., Poss, M., & Pulliam, J. R. C. (2003). Social organization and parasite risk in mammals: Integrating theory and empirical studies. Annual Review of Ecology, Evolution and Systematics, 34, 517–547.
Altmann, J. (1974). Observational study of behavior: Sampling methods. Behaviour, 49, 227–265.
Amato, J. F. R., Amato, S. B., Calegaro-Marques, C., & Bicca-Marques, J. C. (2002). Trypanoxyuris (Trypanoxyuris minutus) associated with the death of a wild southern brown howler monkey, Alouatta guariba clamitans, in Rio Grande Do Sul, Brazil. Arquivos do Instituto Biologico (São Paulo), 69, 99–102.
Anderson, R. M., & May, R. M. (1978). Regulation and stability of host-parasite population interactions. Journal of Animal Ecology, 47, 219–247.
Anderson, R. M., & May, R. M. (1979). Population biology of infectious diseases: Part 1. Nature, 280, 361–367.
Anderson, R. M., & May, R. M. (1991). Infectious diseases of humans. Australian Journal of Public Health, 16, 208–212.
Appleton, C. C., & Henzi, S. P. (1993). Environmental correlates of gastrointestinal parasitism in montane and lowland baboons in Natal, South Africa. International Journal of Primatology, 14, 623–635.
Asensio, N., Cristóbal-Azkarate, J., Dias, P. A. D., Veà, J. J., & Rodríguez-Luna, E. (2007). Foraging habits of mantled howler monkeys (Alouatta palliata mexicana) in three forest fragments. Folia Primatologica, 78, 141–153.
Bogitsh, B. J., Carter, C. E., & Oeltmann, T. N. (2013). Human parasitology. Boston, MA: American Press.
Brunker, K., Hampson, K., Horton, D. L., & Biek, R. (2012). Integrating the landscape epidemiology and genetics of RNA viruses: Rabies in domestic dogs as a model. Parasitology, 139, 1899–1913.
Burnham, K. P., & Anderson, D. R. (2010). Model selection and multi-model inference: A practical information-theoretic approach. New York: Springer Science+Business Media.
Bush, A. O., Lafferty, K. D., Lotz, J. M., & Shostak, A. W. (1997). Parasitology meets ecology own terms: Margolis et al. revisited. Journal of Parasitology, 83, 575–583.
Chapman, C. A., Bowman, D. D., Ghai, R. R., Gogarten, J. F., Goldberg, T. L., Rothman, J. M., Twinomugisha, D., & Walsh, C. (2012). Protozoan parasites in group-living primates: Testing the biological island hypothesis. American Journal of Primatology, 74, 510–517.
Chapman, C. A., & Chapman, L. J. (2000). Determinants of group size in primates: The importance of travel costs. In S. Boinski & P. A. Garber (Eds.), On the move: How and why animals travel in groups (pp. 24–42). Chicago: Chicago University Press.
Chapman, C. A., Gillespie, T. R., & Speirs, M. L. (2005). Parasite prevalence and richness in sympatric colobines: Effects of host density. American Journal of Primatology, 67, 259–266.
Chapman, C. A., Hodder, S. A. M., & Rothman, J. M. (2009). Host-parasite dynamics: connecting primate field data to theory. In M. A. Huffman & C. A. Chapman (Eds.), Host-parasite dynamics: Connecting primate field data to theory (pp. 463–483). Cambridge, UK: Cambridge University Press.
Chapman, C. A., Saj, T. L., & Snaith, T. V. (2007). Temporal dynamics of nutrition, parasitism and stress in Colobus monkeys: Implication for population regulation and conservation. American Journal of Physical Anthropology, 134, 240–250.
Chapman, C. A., Wrangham, R., & Chapman, L. J. (1995). Ecological constraints on group size: An analysis of spider monkey and chimpanzee subgroups. Behavioural Ecology and Sociobiology, 36, 59–70.
Clay, C. A., Lehmer, E. M., Previtali, A., St Jeor, S., & Dearing, M. D. (2009). Contact heterogeneity in deermice: Implications for Sin Nombre virus transmission. Proceedings of the Royal Society B: Biological Sciences, 276, 1305–1312.
Clough, D., Heistermann, M., & Kappeler, P. M. (2010). Host intrinsic determinants and potential consequences of parasite infection in free-ranging red-fronted lemurs (Eulemur fulvus rufus). American Journal of Physical Anthropology, 142, 441–452.
Condit, R., Ashton, P. S., Baker, P., Bunyavejchewin, S., Gunatilleke, S., Gunatilleke, N., Hubbell, S. P., Foster, R. B., Itoh, A., LaFrankie, J. V., Lee, H. S., Losos, E., Manokaran, N., Sukumar, R., & Yamakura, T. (2000). Spatial patterns in the distribution of tropical tree species. Science, 288, 1414–1418.
Cook, G. C. (1994). Enterobius vermicularis infection. Gut, 35, 1159–1162.
Cortés-Ortiz, L., Duda, T. F., Canales-Espinosa, D., García-Orduña, F., Rodríguez-Luna, E., & Bermingham, E. (2007). Hybridization of large-bodied New World primates. Genetics, 176, 2421–2425.
Dias, P. A. D., Alvarado-Serrano, D., Rangel-Negrín, A., Canales-Espinosa, D., & Cortés-Ortiz, L. (2013). Landscape attributes affecting the natural hybridization of Mexican howler Monkeys. In L. K. Marsh & C. A. Chapman (Eds.), Primates in fragments: Complexity and resilience (pp. 423–435). New York: Springer Science+Business Media.
Dias, P. A. D., & Rangel-Negrín, A. (in press). Diets of howler monkeys. In M. Kowalewski, P. A. Garber, L. Cortés-Ortiz, B. Urbani, & D. Youlatos (Eds.), Howler monkeys. Developments in Primatology: Progress and Prospects. New York: Springer Science+Business Media.
Dias, P. A. D., Rangel-Negrín, A., Veà, J. J., & Canales-Espinosa, D. (2010). Coalitions and male-male behavior in Alouatta palliata. Primates, 51, 91–94.
Dias, P. A. D., & Rodríguez-Luna, E. (2005). Organización espacial y dominancia social en machos Alouatta palliata en la isla de Agaltepec, Veracruz, Mexico. Universidad y Ciencia, Número especial II, 45–53.
Dias, P. A. D., Rodríguez-Luna, E., & Canales-Espinosa, D. (2008). The greeting ceremony of mantled howlers (Alouatta palliata): Functions of ritual behavior among males in a Neotropical primate. American Journal of Primatology, 70, 621–628.
Di Fiore, A., & Campbell, C. J. (2007). The atelines: Variation in ecology, behavior, and social organization. In C. J. Campbell, A. Fuentes, K. C. MacKinnon, M. Panger, & S. K. Bearder (Eds.), Primates in perspective (pp. 155–185). New York: Oxford University Press.
Dobson, A. P., & Meagher, M. (1996). The population dynamics of brucellosis in the Yellowstone National Park. Ecology, 77, 1026–1036.
Dunn, J. C., Asensio, N., Arroyo-Rodríguez, V., Schnitzer, S., & Cristóbal-Azkarate, J. (2012). The ranging costs of a fallback food: Liana consumption supplements diet but increases foraging effort in howler monkeys. Biotropica, 44, 705–714.
East, I. J., & Bourne, A. S. (1988). A comparison of worm burden and fecal egg count for measuring the efficacy of vaccination against Oesophagostomum radiatum. International Journal for Parasitology, 18, 863–864.
Estrada, A. (1984). Resource use by howler monkeys (Alouatta palliata) in the rain forest of Los Tuxtlas, Veracruz, Mexico. International Journal of Primatology, 5, 105–131.
Ezenwa, V. O. (2003). Habitat overlap and gastrointestinal parasitism in sympatric African bovids. Parasitology, 126, 379–288.
Ezenwa, V. O. (2004). Host social behavior and parasitic infection: A multifactorial approach. Behavioral Ecology, 15, 446–454.
Ezenwa, V. O., Price, S. A., Altizer, S., Vitone, N. D., & Cook, K. C. (2006). Host traits and parasite species richness in even and odd-toed hoofed mammals, Artiodactyla and Perissodactyla. Oikos, 115, 526–536.
Fenner, A. L., Godfrey, S. S., & Bull, C. M. (2011). Using social networks to deduce whether residents or dispersers spread parasites in a lizard population. Journal of Animal Ecology, 80, 835–843.
Freeland, W. J. (1976). Pathogens and the evolution of primate sociality. Biotropica, 8, 12–24.
Freeland, W. J. (1979). Primate social groups as biological islands. Ecology, 60, 719–728.
Freeland, W. J. (1980). Mangabey (Cercocebus albigena) movement patterns in relation to food availability and fecal contamination. Ecology, 61, 1297–1303.
Gilbert, K. A. (1994). Endoparasitic infection in red howling monkeys (Alouatta seniculus) in the central Amazonian Basin: A cost of sociality? Ph.D. thesis, State University of New Jersey.
Gillespie, T. R., & Chapman, C. A. (2008). Forest fragmentation, the decline of an endangered primate, and changes in host-parasite interactions relative to an unfragmented forest. American Journal of Primatology, 70, 222–230.
Gillespie, T. R., Chapman, C. A., & Greiner, E. C. (2005). Effects of logging on gastrointestinal parasite infections and infection risk in African primates. Journal of Applied Ecology, 42, 699–707.
Glander, K. E. (1978). Howling monkey feeding behaviour and plant secondary compounds: A study of strategies. In G. G. Montgomery (Ed.), Howling monkey feeding behaviour and plant secondary compounds: A study of strategies (pp. 561–574). Washington, DC: Smithsonian Institution Press.
Godfrey, S. S., Bull, C. M., James, R., & Murray, K. (2009). Network structure and parasite transmission in a group living lizard, the gidgee skink, Egernia stokesii. Behavioral Ecology and Sociobiology, 63, 1045–1056.
Gompper, E. M. (2004). Correlations of coati (Nasua narica) social structure with parasitism by ticks and chiggers. In V. Sánchez-Cordero & R.A. Medellín (Eds.), Contribuciones Mastozoológicas en homenaje a Bernardo Villa (pp. 527–534). Instituto de Biología e Instituto de Ecología, México: UNAM Press.
González-Hernández, M., Dias, P. A. D., Romero-Salas, D., & Canales-Espinosa, D. (2011). Does home range use explain the relationship between group size and parasitism? A test with two sympatric species of howler monkeys. Primates, 52, 211–216.
Griffin, R. H., & Nunn, C. L. (2012). Community structure and the spread of infectious disease in primate social networks. Evolutionary Ecology, 26, 779–800.
Gulland, F. M. D. (1992). The role of nematode parasites in Soay sheep (Ovis aries L.) mortality during a population crash. Parasitology Research, 105, 493–503.
Hodder, S. A. M., & Chapman, C. A. (2012). Do nematode infections of red colobus (Procolobus rufromitratus) and black-and-white colobus (Colobus guereza) on humanized forest edges differ from those on nonhumanized forest edges? International Journal of Primatology, 33, 845–859.
Huffman, M. A., & Chapman, C. A. (2009). Primate parasite ecology. Cambridge Studies in biological and Evolutionary Anthropology 57. Cambridge, UK: Cambridge University Press.
Johnson, M. B., Lafferty, K. D., van Oosterhout, C., & Cable, J. (2011). Parasite transmission in social interacting hosts: Monogenean epidemics in guppies. PLoS ONE, 6(8), e22634.
Klein, S. L. (2004). Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunology, 26, 247–264.
Kowalzik, B. K., Pavelka, M. S. M., Kutz, S. J., & Behie, A. (2010). Parasites, primates and ant-plants: Clues to the life cycle of Controrchis spp. in black howler monkeys (Alouatta pigra) in southern Belize. Journal of Wildlife Diseases, 46, 1330–1334.
Lehman, J., Korstjens, A. H., & Dunbar, R. I. M. (2007). Group size, grooming, and social cohesion in primates. Animal Behaviour, 74, 1617–1629.
Levecke, B., Behnke, J. M., Ajjampur, S. S. R., Albonico, M., Ame, S. M., Charlier, J., Stefan, M., Geiger, S. M., Hoa, N. T. V., Ngassam, R. I. K., Kotze, A. C., McCarthy, J. S., Montresor, A., Periago, M. V., Roy, S., Tchuente, L. A. T., Thach, D. T. C., & Vercruysse, J. (2011). A comparison of the sensitivity and fecal egg counts of the McMaster egg counting and Kato-Katz thick smear methods for soil-transmitted helminths. PLOS Neglected Tropical Diseases, 5, e1201.
Lloyd-Smith, J. O., Schreiber, S. J., Kopp, P. E., & Getz, W. M. (2005). Superspreading and the effect of individual variation on disease emergence. Nature, 438, 355–359.
MacIntosh, A. J. J., Hernandez, A. D., & Huffman, M. A. (2010). Host age, sex, and reproductive seasonality affect nematode parasitism in wild Japanese macaques. Primates, 51, 353–364.
MacIntosh, A. J. J., Jacobs, A., García, C., Shimizu, K., Mouri, K., Huffman, M. A., & Hernandez, A. D. (2012). Monkeys in the middle: Parasite transmission through the social network of a wild primate. Plos ONE, 7, e1144.
Mbora, D. N. M., Wieczkowski, J., & Munene, E. (2009). Links between habitat degradation, and social group size, fecundity, and parasite prevalence in the Tana River Mangabey (Cercocebus galeritus). American Journal of Physical Anthropology, 140, 562–571.
McCallum, H., Barlow, N., & Hone, J. (2001). How should pathogen transmission be modeled? Trends in Ecology and Evolution, 16, 295–300.
Meade, B. J. (1984). Host-parasite dynamics among Amboseli baboons. Ph.D. thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA.
Milton, K. (1980). The foraging strategy of howler monkeys: A study of primate economics. New York: Columbia University Press.
Moore, J. (2002). Parasites and the behavior of animals. Oxford: Oxford University Press.
Motulsky, H. J., & Christopoulos, A. (2003). Fitting models to biological data using linear and nonlinear regression. A practical guide to curve fitting. San Diego: GraphPad Software Inc.
Møller, A. P., Dufva, R., & Allander, K. (1993). Parasites and the evolution of host social behavior. Advances in the Study of Behavior, 22, 65–102.
Muehlenbein, M. P. (2005). Parasitological analyses of the male chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. American Journal of Primatology, 65, 167–179.
Muehlenbein, M. P., & Watts, D. P. (2010). The costs of dominance: Testosterone, cortisol, and intestinal parasites in wild male chimpanzees. BioPsychoSocial Medicine, 4, 21.
Nunn, C. L., & Altizer, S. M. (2004). Sexual selection, behaviour and sexually transmitted diseases. In P. M. Kappeler & C. P. van Schaik (Eds.), Sexual selection in primates: New and comparative perspectives (pp. 117–130). Cambridge, UK: Cambridge University Press.
Nunn, C. L., & Altizer, S. M. (2006). Infectious diseases in primates: Behavior, ecology and evolution. Oxford: Oxford University Press.
Nunn, C. L., Altizer, S. M., Sechrest, W., Jones, K. E., Barton, R. A., & Gittleman, J. L. (2004). Parasite species richness and the evolutionary diversity of primates. American Naturalist, 164, S90–S103.
Nunn, C. L., & Dokey, A. T. W. (2006). Ranging patterns and parasitism in primates. Biology Letters, 2, 351–354.
Nunn, C. L., Thrall, P. H., Leendertz, F. H., & Boesch, C. (2011). The spread of fecally transmitted parasites in socially-structured populations. PLoS ONE, 6, e21677.
Oates, J. F. (1977). The guereza and its food. In T. H. Clutton-Brock (Ed.), Primate ecology: Studies of feeding and ranging behaviour in lemurs, monkeys and apes (pp. 275–321). New York: Academic Press.
Osorio, D., García-Hernández, J., García-Prieto, L., & Romero-Callejas, E. (2009). Métodos de diagnóstico parasicológico mediante técnicas no invasivas en antropoides no humanos (pp. 1–10). Instituto de Biología, Mexico City: UNAM Press.
Roberts, J. L., & Swan, R. A. (1981). Quantitative studies of bovine haemonchosis. I. Relationship between fecal egg counts and total worm counts. Veterinary Parasitology, 8, 165–171.
Rodríguez-Luna, E., Solórzano-García, B., Shedden, A., Rangel-Negrín, A., Dias, P. A. D., Cristóbal-Azkárate, J., Cortés-Ortiz, L., Dunn, J. C., Domingo-Balcells, C., Sánchez, S., Vea-Baró, J., & Cornejo, J. (2009). Taller de conservación, análisis y manejo planificado para los primates mexicanos, 2006. CBSG: Universidad Veracruzana.
Schülke, O., & Ostner, J. (2012). Ecological and social influences on sociality. In J. Mitani, J. Call, P. Kappeler, R. Palombit, & J. Silk (Eds.), The evolution of primate societies (pp. 195–219). Chicago: University of Chicago Press.
Seivwright, L. J., Redpath, S. M., Mougeot, F., Watt, L., & Hudson, P. J. (2004). Faecal egg counts provide a reliable measure of Trichostrongylus tenuis intensities in free-living red grouse Lagopus lagopus scoticus. Journal of Helminthology, 78, 69–76.
Setchell, J. M., Bedjabaga, I.-B., Goosens, B., Reed, P., Wickings, E. J., & Knapp, L. A. (2007). Parasite prevalence, abundance, and diversity in a semi-free-ranging colony of Mandrillus sphinx. International Journal of Primatology, 28, 1345–1362.
Shirley, M. D. F., & Rushton, S. P. (2005). Where diseases and networks collide: Lessons to be learnt from a study of the 2001 foot-and-mouth disease epidemic. Epidemiology and Infection, 133, 1023–1032.
Snaith, T. V., Chapman, C. A., Rothman, J. M., & Wasserman, M. D. (2008). Bigger groups have fewer parasites and similar cortisol levels: A multi-group analysis in red colobus monkeys. American Journal of Primatology, 70, 1–9.
Stear, M. J., Bishop, S. C., Doligalska, M., Duncan, J., Holmes, P. H., Irvine, J., McCririe, L., Mckellar, Q. A., Sinski, E., & Murray, M. (1995). Regulation of egg production, worm burden, worm length and worm fecundity by host responses in sheep infected with Ostertagia circumcincta. Parasite Immunology, 17, 643–652.
Stoner, K. E. (1996). Prevalence and intensity of intestinal parasites in mantled howling monkeys (Alouatta palliata) in northeastern Costa Rica: Implications for conservation. Conservation Biology, 10, 539–546.
Stuart, M. D., Greenspan, L. L., Glander, K. E., & Clarke, M. R. (1990). A coprological survey of parasites of wild mantled howling monkeys, Alouatta palliata palliata. Journal of Wildlife Diseases, 25, 547–549.
Stuart, M., Pendergast, V., Rumfelt, S., Pierberg, S., Greenspan, L., Glander, K., & Clarke, M. (1998). Parasites of wild howlers (Alouatta spp.). International Journal of Primatology, 19, 493–512.
Tompkins, D. M., Dunn, A. M., Smith, M. J., & Telfer, S. (2011). Wildlife diseases: From individuals to ecosystems. Journal of Animal Ecology, 80, 19–38.
Trejo-Macías, G., & Estrada, A. (2011). Trypanoxyuris (trypanoxyuris) minutus (Nematoda: Oxyuridae) en las dos especies de monos aulladores (Cebidae) de México. Revista Mexicana de Biodiversidad, 82, 293–299.
Trejo-Macías, G., Estrada, A., & Mosqueda-Cabrera, M. A. (2007). Survey of helminth parasites in populations of Alouatta palliata mexicana and A. pigra in continuous and in fragmented habitat in southern Mexico. International Journal of Primatology, 28, 931–945.
Utzinger, J., Booth, M., N’goran, E. K., Müller, I., Tanner, M., & Lengeler, C. (2001). Relative contribution of day-to-day and intra-specimen variation in faecal egg counts of Schistosoma mansoni before and after treatment with praziquantel. Parasitology, 122, 537–544.
Van Belle, S., Estrada, A., Ziegler, T. E., & Strier, K. B. (2009). Sexual behavior across ovarian cycles in wild black howler monkeys (Alouatta pigra): Male mate guarding and female mate choice. American Journal of Primatology, 71, 153–164.
Vitazkova, S. K. (2009). Overview of parasites infecting howler monkeys, Alouatta sp., and potential consequences of human-howler interactions. In M. A. Huffman & C. A. Chapman (Eds.), Primate parasite ecology. Cambridge Studies in biological and Evolutionary Anthropology 57 (pp. 371–386). Cambridge, UK: Cambridge University Press.
Vitone, N. D., Altizer, S., & Nunn, C. L. (2004). Body size, diet and sociality influence species richness of parasitic worms in anthropoid primates. Evolutionary Ecology Research, 6, 183–199.
Wilson, K., Bjørnstad, O. N., Dobson, A. P., Merler, S., Poglayen, G., Randolph, S. E., Read, A. F., & Skorping, A. (2002). Heterogeneities in macroparasite infections: patterns and processes. In P. J. Hudson, A. Rizzoli, B. T. Grenfell, H. Heesterbeek, & A. P. Dobson (Eds.), The Ecology of Wildlife Diseases (pp. 6–44). Oxford: Oxford University Press.
Zuk, M., & McKean, K. A. (1996). Sex differences in parasite infections: Patterns and processes. International Journal of Parasitology, 26, 1009–1024.
Acknowledgments
We thank Roger Pérez for permission to conduct this research on his ranch and Liliana Cortés Ortiz for background information on the study groups; A. Coyohua, D. Medrano, A. Droussin, F. Burnonville, G. Muntané, M. G. Cárdenas, and A. Sánchez for field assistance; S. Sinaca-Colín for helping with the identification of plant species; D. Osorio and J. García for helping in parasite identification; D. Romero for providing laboratory facilities; and C. Schaffner, F. Aureli, J. Setchell, and three anonymous reviewers for their comments that greatly improved the manuscript. This study was financed and supported by CONACyT (MGH scholarship no. 229901; i010/458/2013 C-703/2013), Idea Wild, and Universidad Veracruzana. P. A. D. Dias and A. Rangel-Negrín thank Mariana for insights into primate behavior and health.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Individual results on Trypanoxyuris minutus for sympatric Alouatta palliata and A. pigra in Tabasco, Mexico (Appendix S1) are available online.
Appendix S1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
González-Hernández, M., Rangel-Negrín, A., Schoof, V.A.M. et al. Transmission Patterns of Pinworms in Two Sympatric Congeneric Primate Species. Int J Primatol 35, 445–462 (2014). https://doi.org/10.1007/s10764-014-9751-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-014-9751-y