Abstract
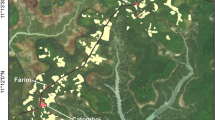
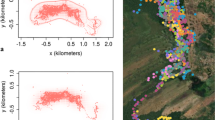
The predicted relationship between home-range size and group mass in primates developed by Clutton-Brock and Harvey (1977) has proved extremely robust in describing the use of space by most primate species. However, mandrills (Mandrillus sphinx) are now known to have an extreme group mass in the wild, far larger than that of the species used originally to generate that relationship, and so it was unknown whether this relationship would be robust for this species. We investigated the home-range size and use of a wild horde of ca. 700 mandrills in Lopé National Park, Gabon, using radiotelemetry. The total area the horde used over a 6-yr period [100% minimum convex polygon (MCP)] was 182 km2, including 89 km2 of suitable forest habitat. Mandrills used gallery forests and isolated forest fragments with high botanical diversity far more intensively that the continuous forest and completely avoided savanna and marsh. Peeled polygons and fixed kernel contours revealed multiple centres of use, with the horde spending more than half its time in <10% of the total documented range, typical of a frugivore using a patchy environment. Home-range size and internal structure varied considerably between years, but total home range fitted the predicted relationship between group mass and home range size, despite being an outlier to the dataset. We discuss the conservation implications of the species’ space requirements, in light of current pressures on land use in their range.






Similar content being viewed by others
References
Abernethy, K. A., & White, L. J. T. (2010). Mandrillus sphinx. In T. Butynski, D. Happold, & J. Kingdon (Eds.), Mammals of Africa. San Francisco: University of California Press, in press.
Abernethy, K. A., White, L. J. T., & Wickings, E. J. (2002). Hordes of mandrills (Mandrillus sphinx): Extreme group size and seasonal male presence. Journal of Zoology (London), 258, 131–137.
Altmann, S. A. (1974). Baboons, space, time and energy. American Zoologist, 14, 221–248.
Amlaner, C. J., Jr., & Macdonald, D. W., eds. (1980). A handbook on biotelemetry and radiotracking. Proceedings of an international conference on telemetry and radio tracking in biology and medicine. Oxford: Pergamon.
Astaras, C., Muhlenberg, M., & Waltert, M. (2008). Note on drill (Mandrillus leucophaeus) ecology and conservation status in Korup National Park, Southwest Cameroon. American Journal of Primatology, 70, 306–310.
Barton, R. A., Whiten, A., Strum, S. C., Byrne, R. W., & Simpson, A. J. (1992). Habitat use and resource availability in baboons. Animal Behaviour, 43, 831–844.
Boinski, S. (1987). Habitat use by squirrel monkeys (Saimiri oerstedi) in Costa Rica. Folia Primatologica, 49, 151–167.
Bowen-Jones, E., & Pendry, S. (1999). The threat to primates and other mammals from the bushmeat trade in Africa, and how this threat could be diminished. Oryx, 33, 233–246.
Cade, B. S., & Richards, J. D. (2001). User manual for BLOSSOM statistical software. Fort Collins: Midcontinent Ecological Science Centre, US Geological Survey.
Chapman, C. A. (1988). Patterns of foraging and range use by three species of neotropical primates. Primates, 29, 177–194.
Chapman, C. A., Wrangham, R. W., Chapman, L. J., Kennard, D. K., & Zanne, A. E. (1999). Fruit and flower phenology at two sites in Kibale National Park, Uganda. Journal of Tropical Ecology, 15, 189–211.
Chapman, C. A., Chapman, L. J., Struhsaker, T. T., Zanne, A. E., Clark, C. J., & Poulsen, J. R. (2005). A long-term evaluation of fruiting phenology: Importance of climate change. Journal of Tropical Ecology, 21, 31–45.
Chapman, C. A., Lawes, M. J., & Eeley, H. A. C. (2006). What hope for African primate diversity? African Journal of Ecology, 44, 116–133.
Clutton-Brock, T. H. (1977). Primate ecology: Studies of feeding and ranging behaviour in lemurs, monkeys and apes. London: Academic Press.
Clutton-Brock, T. H., & Harvey, P. H. (1977). Primate ecology and social organisation. Journal of Zoology (London), 183, 1–29.
Cowlishaw, G. (1997). Trade-offs between foraging and predation risk determine habitat use in a desert baboon population. Animal Behaviour, 53, 667–686.
De Solla, S. R., Bonduriansky, R., & Brooks, R. J. (1999). Eliminating autocorrelation reduces biological relevance of home range estimates. The Journal of Animal Ecology, 68, 221–234.
Defler, T. R. (1996). Aspects of the ranging pattern in a group of wild woolly monkeys (Lagothrix lagothricha). American Journal of Primatology, 38, 289–302.
Di Bitetti, M. S. (2001). Home-range use by the tufted capuchin monkey (Cebus apella nigritus) in a subtropical rainforest of Argentina. Journal of Zoology (London), 253, 33–45.
Fa, J. E., Juste, J., Delval, J. P., & Castroviejo, J. (1995). Impact of market hunting on mammal species in Equatorial Guinea. Conservation Biology, 9, 1107–1115.
Freeland, W. J. (1980). Mangabey (Cercocebus albigena) movement patterns in relation to food availability and faecal contamination. Ecology, 61, 1297–1304.
Gadsby, E. L., Jenkins, P. D., Jr., & Feistner, A. T. C. (1994). Coordinating conservation for the drill (Mandrillus leucophaeus): Endangered in forest and zoo. In P. J. S. Olney, G. M. Mace, & A. T. C. Feistner (Eds.), Creative conservation. Interactive management of wild and captive animals (pp. 39–454). London: Chapman and Hall.
Hamilton, W. J. I. (1982). Baboon sleep site preferences and relationships to primate grouping patterns. American Journal of Primatology, 3, 41–53.
Harris, S., Cresswell, W. J., Forde, P. G., Trewhella, W. J., Woollard, T., & Wray, S. (1990). Home-range analysis using radio-tracking data—a review of problems and techniques particularly as applied to the study of mammals. Mammal Review, 20, 97–123.
Harrison, M. J. S. (1988). The mandrill in Gabon's rain forest—ecology, distribution and status. Oryx, 22, 218–228.
Hayne, D. W. (1949). Calculation of size of home range. Journal of Mammalogy, 30, 1–18.
Hladik, C. M. (1975). Ecology, diet, and social patterning in Old and New World primates. In R. H. Tuttle (Ed.), Socio-ecology and psychology of primates (pp. 3–36). The Hague: Mouton.
Hoshino, J. (1985). Feeding ecology of mandrills (Mandrillus sphinx) in Campo Animal Reserve, Cameroon. Primates, 26, 248–273.
Hoshino, J., Mori, A., Kudo, H., & Kawai, M. (1984). Preliminary report on the grouping of mandrills (Mandrillus sphinx) in Cameroon. Primates, 25, 295–307.
IUCN (2009). 2009 IUCN Red List of Threatened Species. www.iucnredlist.org.
Jenrich, R. I., & Turner, F. B. (1969). Measurement of non-circular home range. Journal of Theoretical Biology, 22, 227–237.
Jouventin, P. (1975). Observations sur la socio-ecologie du mandrill. Terre et Vie, 29, 439–532.
Kaplin, B. A. (2001). Ranging behavior of two species of guenons (Cercopithecus lhoesti and C. mitis doggetti) in the Nyungwe Forest Reserve, Rwanda. International Journal of Primatology, 22, 521–548.
Karesh, W. B., Wallace, R. B., Painter, R. L. E., Rumiz, D., Braselton, W. E., Dierenfeld, E. S., et al. (1998). Immobilization and health assessment of free-ranging black spider monkeys (Ateles paniscus chamek). American Journal of Primatology, 44, 107–123.
Kenward, R. E. (1985). Ranging behaviour and population dynamics in grey squirrels. In R. M. Sibly & R. H. Smith (Eds.), Telemetric studies of vertebrates (pp. 319–330). London: Academic Press.
Kenward, R. E. (2001). A manual for wildlife radio tagging. San Diego: Academic Press.
Kernohan, B. J., Gitzen, R. A., & Millspaugh, J. J. (2001). Analysis of animal space use and movements. In J. J. Millspaugh & J. M. Marzluff (Eds.), Radio tracking and animal Populations (pp. 125–166). San Diego: Academic Press.
Lahm, S. A. (1986). Diet and habitat preference of Mandrillus sphinx in Gabon—implications of foraging strategy. American Journal of Primatology, 11, 9–26.
Léal, M. E. (2004). The African rain forest during the Last Glacial Maximum, an archipelago of forests in a sea of grass. PhD thesis Wageningen University, Wageningen, Netherlands.
Mielke, P. W., Jr., & Berry, K. J. (1982). An extended class of permutation techniques for matched pairs. Communications in Statistics A—Theory and. Methods, 11, 1197–1207.
Mielke, P. W., Jr., & Berry, K. J. (2001). Permutation methods: A distance function approach. New York: Springer-Verlag.
Milton, K., & May, M. L. (1976). Body weight, diet and home range area in primates. Nature, 259, 459–462.
Mitani, M. (1989). Cercocebus torquatus—Adaptive feeding and ranging behaviors related to seasonal fluctuations of food resources in the tropical rain-forest of southwestern Cameroon. Primates, 30, 307–323.
Mohr, C. O. (1947). Table of equivalent populations of North American small mammals. American Midland Naturalist, 37, 223–449.
Nasi, R., Brown, D., Wilkie, D., Bennett, E., Tutin, C., van Tol, G., et al. (2008). Conservation and use of wildlife-based resources: The bushmeat crisis. CBD Technical Series no. 33. Montreal: Secretariat of the Convention on Biological Diversity and Bogor and Center for International Forestry Research.
Oates, J. F. (1996). African Primates: Status survey and Conservation Action Plan, Revised Edition. Gland, Switzerland: IUCN/Species Survival Commission (SSC), Primate Specialist Group.
Oates, J. F., & Butynski, T. M. (2008a). Mandrillus sphinx. In: IUCN 2009. IUCN Red List of Threatened Species. Version 2009.2. www.iucnredlist.org.
Oates, J. F., & Butynski, T. M. (2008b). Mandrillus leucophaeus. In: IUCN 2009. IUCN Red List of Threatened Species. Version 2009.2. www.iucnredlist.org.
Olupot, W., Chapman, C. A., Waser, P. M., & Isabirye Basuta, G. (1997). Mangabey (Cercocebus albigena) ranging patterns in relation to fruit availability and the risk of parasite infection in Kibale National Park, Uganda. American Journal of Primatology, 43, 65–78.
Oslisly, R. (2001). The history of human settlement in the middle Ogooue´ valley (Gabon). Implication for the environment. In W. Webber, L. J. T. White, A. Vedder, & L. Naughton-Treves (Eds.), African rain forest ecology & conservation (pp. 101–118). New Haven: Yale University Press.
Otis, D. L., & White, G. C. (1999). Autocorrelation of location estimates and the analysis of radiotracking data. Journal of Wildlife Management, 63, 1039–1044.
Rogers, M. E., Abernethy, K. A., Fontaine, B., Wickings, E. J., White, L. J. T., & Tutin, C. E. G. (1996). Ten days in the life of a mandrill horde in the Lopé Reserve, Gabon. American Journal of Primatology, 40, 297–313.
Sabater Pi, J. (1972). Contribution to the ecology of Mandrillus sphinx Linnaeus 1758 of Rio Muni (Republic of Equatorial Guinea). Folia Primatologica, 17, 304–319.
Sayer, J. A., Harcourt, C. S., & Collins, N. M. (1992). The conservation atlas of tropical Forests: Africa. London: Macmillan.
Seaman, D. E., & Powell, R. A. (1996). An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology, 77, 2075–2085.
Seaman, D. E., Millspaugh, J. J., Kernohan, B. J., Brundige, G. C., Raedeke, K. J., & Gitzen, R. A. (1999). Effects of sample size on kernel home range estimates. Journal of Wildlife Management, 63, 739–747.
Silverman, B. W. (1986). Density estimation for statistics and data analysis. London: Chapman and Hall.
Singleton, I., & van Schaik, C. P. (2001). Orangutan home range size and its determinants in a Sumatran swamp forest. International Journal of Primatology, 22, 877–911.
Terborgh, J. (1983). Five New World Primates: A study in comparative ecology. Princeton, NJ: Princeton University Press.
Tutin, C. E. G., & White, L. J. T. (1998). Primates, phenology and frugivory: Present, past and future patterns in the Lopé reserve, Gabon. In: D. M. Newberry, H. H. T. Prins, & N. Brown (Eds.), Dynamics of tropical communities. 37th Symposium of the British Ecological Society (pp. 309–337). Oxford: Blackwell Science.
Tutin, C. E. G., Fernandez, M., Rogers, M. E., Williamson, E. A., & McGrew, W. G. (1991). Foraging profiles of sympatric gorillas and chimpanzees in the Lopé Reserve, Gabon. Philosophical Transactions of the Royal Society, London (B), 334, 179–186.
Tutin, C. E. G., White, L. J. T., & Mackanga-Missandzou, A. (1997). The use by rain forest mammals of natural forest fragments in an equatorial African savanna. Conservation Biology, 11, 1190–1203.
Ukizintambara, T., White, L., Abernethy, K., & Thébaud, C. (2007). Gallery forests versus bosquets: Conservation of natural fragments at Lopé National Park in central Gabon. African Journal of Ecology, 45, 476–482.
van Schaik, C. P., Terborgh, J. W., & Wright, S. J. (1993). The phenology of tropical forests: Adaptive significance and consequences for primary consumers. Annual Review of Ecology and Systematics, 24, 353–377.
Van Winkle, W. (1975). Comparison of several probabilistic home-range models. Journal of Wildlife Management, 39, 118–123.
Wallace, R. B. (2006). Seasonal variations in black-faced black spider monkey (Ateles chamek) habitat use and ranging behaviour in a southern Amazonian tropical forest. American Journal of Primatology, 68, 313–332.
Walsh, P. D., Abernethy, K. A., Bermejo, M., Beyersk, R., De Wachter, P., Akou, M. E., et al. (2003). Catastrophic ape decline in western equatorial Africa. Nature, 422, 611–614.
White, L. J. T. (1992). Vegetation history and logging damage; effects on rain forest mammals in the Lopé Reserve. Gabon: Doctoral thesis, University of Edinburgh, Edinburgh.
White, L. J. T. (1995). Vegetation study of Lopé reserve. Gabon: Unpublished report, ECOFAC.
White, L. J. T. (2001). Forest-savanna dynamics and the origins of Marantaceae forest in Central Gabon. In W. Weber, L. J. T. White, A. Vedder, & L. Naughton-Treves (Eds.), African rain forest ecology and conservation: An interdisciplinary perspective (pp. 165–182). New Haven and London: Yale University Press.
White, E. C. (2007). Ecology of Mandrillus sphinx: Ranging, diet and social structure of a mandrill horde in Lopé National Park. Gabon: Doctoral thesis, University of Exeter, Cornwall, U.K.
White, L. J. T., & Abernethy, K. (1997). A guide to the vegetation of the Lopé Reserve, Gabon. Libreville, Gabon: ECOFAC.
White, G. C., & Garrott, R. A. (1990). Analysis of wildlife radio-tracking data. San Diego: Academic Press.
Wickings, E. J., & Dixson, A. F. (1992). Development from birth to sexual maturity in a semi-free- ranging colony of mandrills (Mandrillus sphinx) in Gabon. Journal of Reproduction and Fertility, 95, 129–138.
Wild, C., Morgan, B. J., & Dixson, A. (2005). Conservation of drill populations in Bakossiland, Cameroon: Historical trends and current status. International Journal of Primatology, 26, 759–773.
Wilkie, D. S., & Laporte, N. T. (2001). Forest area and deforestation in Central Africa. Current knowledge and future directions. In W. Weber, L. J. T. White, A. Vedder, & L. Naughton-Treves (Eds.), African rain forest ecology and conservation: An interdisciplinary perspective (pp. 119–139). New Haven and London: Yale University Press.
Worton, B. J. (1989). Kernel methods for estimating the utilization distribution in home-range studies. Ecology, 70, 164–168.
WRI Global Forest Watch. Interactive Forest Atlas series. (2009). www.wri.org/gfw
Acknowledgments
We thank the Ministère des Eaux et Forêts and the Agence National des Parcs Nationaux (ANPN, formerly CNPN) for permission to work in Lopé National Park. In particular, we thank the late Dr. Alphonse Mackanga-Missandzou, M. Simon Angoué and M. Jean Tondangoye, and M. Augustin Mihindou for support at Lopé as Chefs de Brigade de Faune 1998–2002, and from 2003, M. Joseph Ngowou as National Park conservator. We thank the Centre International de Recherches Médicales de Franceville (CIRMF) for long-term core funding of SEGC, from where the study was carried out, and for support to K. Abernethy. The Wildlife Conservation Society Field Veterinary Program (1998–2003) provided support to W. B. Karesh and M. D. Kock, and Edith McBean and the Disney Foundation (1998–2000) provided project grants to K. Abernethy and L. White via the WCS Africa Program. The Welcome Trust provided support to T. Ukizintambara and the Natural Environment Research Council (NERC), U.K. provided studentship (NER/S/A/2002/12024) and fieldwork expenses grant to E. C. White, via the University of Stirling. We thank ECOFAC II (Gabon) and WCS Gabon for logistical support in the field, and Martial Bouassa, Jean-Gael Emptaz-Collomb, Tom Gilbert, Philipp Henschel, Olivier Houé, Miguel Léal, Ludovic Momont, Jules Penze, Peter Ragg, Trish Reed, Paul Telfer, Caroline Tutin, Peter Walsh, and E. Jean Wickings for their assistance in the field, in particular capturing mandrills for radio-collaring. We also thank David Bryant and 2 anonymous reviewers for useful comments and suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
White, E.C., Dikangadissi, JT., Dimoto, E. et al. Home-range Use by a Large Horde of Wild Mandrillus sphinx . Int J Primatol 31, 627–645 (2010). https://doi.org/10.1007/s10764-010-9417-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-010-9417-3