Abstract
Researchers studying individual variation in conspicuous skin coloration in primates have suggested that color indicates male quality. Although primate fur color can also be flamboyant, the potential condition dependence and thus signaling function of fur remains poorly studied. We studied sources of variation in sexually dichromatic facial hair coloration in red-fronted lemurs (Eulemur fulvus rufus). We collected data on 13 adult males in Kirindy Forest, Madagascar, during two study periods in 2006 and 2007, to determine whether variation in facial hair coloration correlates with male age, rank, androgen status, and reproductive success. We quantified facial hair coloration via standardized digital photographs of each male, assessed androgen status using fecal hormone measurements, and obtained data on reproductive success through genetic paternity analyses. Male facial hair coloration showed high individual variation, and baseline coloration was related to individual androgen status but not to any other parameter tested. Color did not reflect rapid androgen changes during the mating season. However, pronounced long-term changes in androgen levels between years were accompanied by changes in facial hair coloration. Our data suggest that facial hair coloration in red-fronted lemur males is under proximate control of androgens and may provide some information about male quality, but it does not correlate with dominance rank or male reproductive success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many primate species males and females can be distinguished by striking color differences. Such sexual dichromatism ranges from whole-body coloration, e.g., silver backs in male gorillas, to locally restricted areas such as whiskers, blazes, or ocular markings (Dixson 1998; Gerald 2003). In contrast to interspecific color differences, which may function in species recognition or concealment (Andersson 1994; Bradley and Mundy 2008), color ornaments that differ between the sexes carry the potential to convey quality information to mating partners or to competitors of the same sex (Andersson 1994). Coloration is under complex regulatory control by multiple distinct factors that interact at different levels to determine pigmented phenotype (Hearing 1999; Slominski et al. 2004a). This makes colored ornaments potentially more malleable than other visual ornaments, facilitating the conveyance of various kinds of quality information that may be of particular interest in intra- and intersexual competition. Theoretical work and research on other vertebrates have demonstrated that colored ornaments carry costs, e.g., increased predation risk, agonistic interactions, or nutritional constraints, so that fully developed ornaments can act as honest signals of individual quality (Folstad and Karter 1992; Hamilton and Zuk 1982; Maynard-Smith and Harper 2003; Rohwer and Ewald 1981; Veiga and Puerta 1996; Zahavi 1975).
Historically, most research on signaling functions of color ornaments in vertebrates has been conducted on birds, fish, and reptiles (Buchholz 1995; Hausfater et al. 1990; Hill 1991; Milinski and Bakker 1990; Ressel and Schall 1989; Wedekind 1992), but recent work has begun to focus on color ornaments as signals in primates. Studies of primate skin coloration have shown that the development of bright coloration can vary with age (Setchell and Dixson 2001b; Setchell et al. 2008), dominance rank (Gerald 2001; Setchell and Wickings 2005), and reproductive state (Bergman and Beehner 2008; Higham et al. 2008) of both males and females. Androgens are indirectly responsible for reddening of facial and anogenital skin in male rhesus macaques via aromatization to estrogens, which in turn increase vascularization and epidermal blood flow (Rhodes et al. 1997; Vandenbergh 1965; Waitt et al. 2003). Similarly, in mandrills Setchell and Wickings (2005) have shown the degree of the red coloration of the facial skin to be androgen related, and it appears to be used as a badge of status. Females of both species showed a preference for the brightest colored males as mating partners (Gerald et al. 2007; Setchell 2005; Waitt et al. 2003), and males seem to judge competing males with regard to their color signal (Setchell and Dixson 2001a; Setchell et al. 2008). These observations strongly suggest that certain primate skin colorations act as ornaments in inter— and intrasexual communication and selection.
However, flamboyant coloration in primates is not restricted to skin color, as sexual dichromatism also occurs in pelage and facial hair coloration, where one sex usually shows more conspicuous coloration than the other (Gerald 2003). Studies of the adaptive significance and physiological correlates of primate hair coloration are rare (Bradley and Mundy 2008), presumably owing to the general view that hair coloration is a permanent cue that does not vary in form or intensity, instead of a signal that conveys quality traits (Gerald 2003; Hauser 1996). However, hair color is under the control of multiple agents including androgens (Slominski et al. 2004a), such that hair color can fluctuate between and within individuals as a function of changes in hormone levels.
Hair color in primates is based on the melanin-based pigments eumelanin and phaeomelanin (Castanet and Ortonne 1996; Slominski et al. 2004a), which are produced in the hair follicle bulb during melanogenesis (Castanet and Ortonne 1996). During the anagen (growth) phase of hair, androgens can affect hair color without complete molt by altering the production of specific regulatory factors in the hair bulb (Randall 2000, 2008). These factors may in turn alter the activity of other follicular cells, including melanocytes, resulting in a shifted ratio of eumelanin to phaeomelanin, affecting hair color development (Slominski et al., 2004a, b). Studies of black lemurs (Eulemur macaco macaco) appear to confirm this physiological underpinning, as manipulation of androgen levels affect hair coloration in both sexes (Asa et al. 2007; A. Yoder, pers.comm. cited in Barthold et al. 2008). These findings are backed by results of studies on lion mane coloration (West and Packer 2002), as well as further evidence from patas monkeys (Erythrocebus patas: Loy 1974; Palmer et al. 1981), showing that hair color can vary individually and temporally with regard to endocrine function. Yet, due to a lack of studies exploring causal relationships, it remains unknown whether primate hair coloration functions as a signal of quality by mirroring changes in the condition of the bearer.
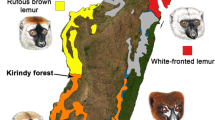
We investigated individual variation in facial hair coloration in red-fronted lemurs (Eulemur fulvus rufus), and explored whether color changes occur within a single individual, a precondition for a possible condition dependency. Eulemur is well known for its sexual dichromatism (Bradley and Mundy 2008; Mittermeier et al. 2006), in which males usually display more striking patterns. In red-fronted lemurs, males exhibit conspicuous facial hair coloration characterized by a rufous crown, white patches around the eyes, a black nasal stripe that extends up from the face dividing the crown, and a uniformly gray body pelage. In contrast, female body pelage is rufous colored and female facial coloration is dominated by dark and white patches of hair (Fig. 1). Male facial coloration varies between individuals with crown color ranging from pale, cream-colored to rufous, bright red color (Fig. 1), and results of an experimental study suggested female Eulemur fulvus sp. prefer the brightest males (Cooper and Hosey 2003). However, there is no information on either the signaling function of hair coloration in red-fronted lemurs or the physiological correlates of this colorful trait under natural conditions. Androgen levels in this species vary according to reproductive season (Ostner et al. 2002, 2008), providing a natural experiment, enabling us to test whether facial crown red hair color of male red-fronted lemurs is androgen dependent; if facial coloration is able to mirror changes in males’ androgen status rapidly, seasonal changes in androgen levels should result in hair color changes in the face.
Old-world anthropoid primates and New World howlers (Alouatta spp.) possess uniform trichromatic vision, wherein both sexes have three divergent photopigment genes (Jacobs 1993; Surridge et al. 2003). Recent studies of primate color vision have provided evidence that along with most New World primates and some diurnal lemur species, red-fronted lemurs, possess a second form of trichromatic vision, known as polymorphic trichromacy (Bradley et al. 2008; Surridge et al. 2003). In contrast to uniform trichromacy, polymorphic trichromacy is based on two photopigment genes, one of which is a polymorphic X-linked gene. Thus heterozygous females can be either dichromatic or trichromatic whereas homozygous females and males can only be dichromatic (Jacobs 1999; Tan and Li 1999). Red and orange reflectance spectra, as seen in male facial coloration, would be cryptic to all males and homozygous females, but conspicuous to heterozygous, trichromatic females (Sumner and Mollon 2003).
Although methods for measuring skin coloration in free-ranging animals are available and used widely in primate skin coloration studies (Bergman and Beehner 2008; Gerald et al. 2001; Higham et al. 2008; Setchell et al. 2006), the quantification of hair color in wild primates, which is characterized by changing reflection properties (Sumner and Mollon 2003), is still in its infancy, and no one has, to our knowledge, measured hair color using digital photography beyond the measurement of luminance (Kamilar, this issue). Therefore we aimed to:
-
1)
adjust and validate methods used to measure animal coloration to assess hair color in a free-ranging primate;
-
2)
explore the signaling function of hair coloration by analyzing variation within and between male red-fronted lemurs in relation to intrinsic variables (age, social rank) and their consequences (reproductive success);
-
3)
investigate whether facial hair color is related to male androgens level under natural conditions both short term (over a period of 4 mo surrounding the mating season) and long term (between two consecutive study periods).
Methods
Study Site and Subjects
We studied red-fronted lemurs at the German Primate Centre (DPZ) field site in Kirindy Forest, a dry deciduous forest in Western Madagascar. The site is managed within a forestry concession operated by the Centre National de Formation, d’Etudes et de Recherche en Environnement et Foresterie (CNFEREF), Morondava. We collected data from all adult males living in four social groups of red-fronted lemurs during two field seasons between March and July in 2006 (n 2006 = 11 males) and 2007 (n 2007 = 13). Of these males, eight were present during both years and could be used for direct comparisons between years; the remaining eight males either left (n = 5) or joined (n = 3) their group in the second year. All individuals were well habituated to human presence and were marked with individual combinations of nylon collars and pendants or radio collars for individual recognition. As part of an ongoing long-term study, one individual per group was equipped with a radio-tag (Biotrack, Wareham, Dorset, UK), which facilitated ad hoc detection of the group.
Red-fronted lemurs live in multimale-multifemale groups and have a polygynandrous mating system. Reproductive skew within a group is usually high, as 1 male per group, the dominant or central male, usually achieves the highest reproductive success (Kappeler and Port 2008; Ostner and Kappeler 1999). During the short mating season (3–4 wk/yr) each female is receptive for only 1–2 d and gestation length lasts ca. 122 d (Izard et al. 1995). Our study periods included the time before, during, and after the mating season, which occurred from mid May to early June in both years.
Collecting Digital Photos
We took photographs of focal males with a Canon EOS 20D digital SLR camera (8.2 megapixels, fitted with a Canon EF 55–200 mm 1:4.5–5.6 II USM lens), which allowed full manual control over metering and exposure. Because the area of interest was the forehead of males, we used full-frontal shots from males that were sitting at eye level of the observer for analysis. We attempted to capture male coloration every week. To guard against major variation in light environment related to time of day and weather conditions (Endler 1993), we restricted data acquisition to a time window from 1100 h to 1400 h on days with blue sky. Accounting for exposure and light differences in the forest required calibration (Gerald et al. 2001). After taking the photographs, we used the sequential method (Bergman and Beehner 2008), which consisted of taking a shot of the standard color chart right after the individual left the spot. We used a qp colorsoft 201 color chart (QPcard AB, Gøteborg, Sweden). Colors on this chart are mixed separately from pigments and are extremely fade resistant, and the chart was also protected from discoloration by storage in a black folder when not in use.
As the preferred forest habitat of red-fronted lemurs is spatially heterogeneous in light intensity, we used a flash (internal EOS 20D flash and external flash Canon speedlite 430EX) to adapt to varying distances and light intensities. We obtained all images within a mean distance of d = 118 (±22.5) cm, and a later analysis of the relationship between distance to object and color score confirmed that these two factors were not related (p = 0.92, linear mixed model [LMM]: dependent variable = distance; fixed covariate = color score; random factor = individual ID). For technical reasons the flash had to be substituted in the second year by an external flash. Testing the integrity of color measurements under altered shooting conditions, we compared repeated measures between years of the color score of five different standard color patches on color charts used in both years. Results of repeated measurement analyses of variance confirmed that color scores did not differ between years and the substitution of the flash did not have a statistically significant effect on color measurements (F 1,174 = 0.00, p = 0.9997, Fig. 3a). We stored all images as 12-bit RAW files and further processed via Adobe Photoshop CS3.
Color Calibration and Measurement
We opened RAW files of paired sets of images of males and matching color standards in Adobe Photoshop CS3 RAW converter and we set CIE temperature to a fixed value of 5400 K, adapting to the ambient light condition at shooting. Because both images were shot using similar manual settings, i.e., similar exposure time and aperture, and because we purposely underexposed all images by 1 or 2 stops in the field to avoid clipping of channels (Bergman and Beehner 2008; Stevens et al. 2007), optimal exposure of images was not possible to achieve in the field and exposure had to be corrected manually using the RAW converter. Starting with the color chart image we corrected the exposure by balancing the color histogram, e.g., exposure + 0.15, and repeated this procedure with the male color image (again exposure + 0.15). This method provided us with optimally exposed color charts; a prerequisite for successful subsequent calibration. Proceeding to the Photoshop surface, we combined matched pairs of male facial portraits and color chart images in a single image and calibrated the combined image following a methodology modified from Gerald et al. (2001): We used the Photoshop adjust level command to reset the combined image to pure white (maximum Red (R), Green (G) and Blue (B) values) as obtained from the color chart. Next we took mean R, G, and B values of the black square on the color chart and reset the image accordingly.
To explore the reliability of our method, we tested 40 corrected images for linearity and RGB equalization, following methods described by Stevens et al. (2007). We used linear regression to analyze the relationship between RGB values measured from the 7-step gray scale on the color chart, and the known nominal reflection values for these seven squares and explored the relationship between measured grayscale value and nominal reflection value for the seven squares in comparison to predicted values.
For analyses of male facial hair coloration, we cropped a standard area of the male’s forehead (60% of the total height) of each corrected image and used the rectangular marquee tool to select 2 measuring areas (Patches) per image, to the left and right of the median axis of the forehead. We measured the color of these 2 patches (left and right) within the RGB color space and obtained average RGB scores for each patch using the Photoshop histogram command. We applied a standardized Principal Component Analyses (PCA; princomp in R) to obtain a single component that explained most of the variability contained in these 3 color channels. The first principal component vector (PC1) explained 98.4% of the variance and provided the quantification of males’ facial coloration, which we refer to as color score.
To compare potential condition dependence of the facial coloration with the coloration of other body parts, we also measured coloration of a gray control patch located on the ruff of males via the same method.
Acquisition of Intrinsic Variables and Reproductive Success
Information on individual social rank was obtained from focal animal behavioral observations of all four groups of red-fronted lemurs (630 observation hours), and evaluated independently for both years. The age of most individuals was known from long-term monitoring of the population. For individuals that had immigrated into the population, we estimated age at first capture via tooth wear and sexual maturity. We obtained information on reproductive success via genetic paternity analyses of infants conceived during the mating season. We collected tissue samples of 16 weaned offspring routinely during an ongoing long-term study and assigned paternities using 11 nuclear markers (microsatellites) developed and established in earlier paternity studies on the same population (Kappeler and Port 2008; Wimmer and Kappeler 2002) and the genetic software CERVUS 2.0. Detailed description of methods can be found in Kappeler and Port (2008).
Hormone Analyses
We determined male androgen status from analyses of fecal samples, which we collected once a week, resulting in 32 samples per focal individual (n total = 512 samples of 16 males in 4 social groups) before, during, and after the mating season. We collected samples directly after defecation from 1100 h–1300 h, the time of day when subjects were most likely to stay close to the ground and individual assignment of feces was straightforward. As it has previously been shown that storage of red-fronted lemur fecal samples in alcohol does not affect fecal androgen concentrations (Ostner et al. 2008), we placed samples in tubes containing 90% ethanol, labeled them, wrapped them with parafilm, and stored them in the shade at ambient temperatures at the field site (25–35°C). At the end of each field season we transported samples to the endocrine laboratory.
Before hormone measurement, we homogenized samples in their original ethanolic solvent by squashing them with a metal stick, including a 3-ml methanolic rinse of the original sample tube, and subsequently extracted twice as described by Ziegler et al. (2000), with the modification that samples were vortex-mixed in each of the 2 extractions for 10 min on a multitube vortexer instead of shaking them overnight on a horizontal shaker. After extraction we dried the remaining fecal pellets in a vacuum oven at 50°C and determined the dry weight of individual samples. We used the supernatant for measurements of immunoreactive testosterone using a microtiter plate enzyme immunoassay (EIA), which researchers have previously validated for monitoring androgen status in lemurs (Kraus et al. 1999; von Engelhardt et al. 2000), including the red-fronted lemur (Ostner et al. 2002). Kraus et al. (1999) described the assay procedure in detail. Sensitivity of the assay at 90% binding was 0.5 pg per well. Intra- and interassay coefficients of variation of high and low value quality controls (QCs) were 6.2% (n = 16) and 7.6% (n = 24; high) and 10.2% (n = 16) and 11.2% (n = 24; low), respectively. QC values from measurements of samples from both years did not differ significantly between years (QChigh: p = 0.191, t 1,22 = 1.348; QClow: p = 0.64, t t 1,22 = −0.475). All hormone values are expressed as mass per gram of dry weight (ng/g).
Statistical Analyses
We collected a total of 112 images of 15 individual males in 2006 and 2007. Images were distributed over time with 16 images taken during the premating period (4 wks before mating), 28 images during the mating period, 26 images 4 wk after, and 30 images 8 wk after the mating period. However, due to unbalanced sample sizes between individuals, we used different analyses and datasets to address our study aims as follows:
-
1)
We conducted validation of the color assessment method, using a data set of images collected during main data collection in the field. For each of 6 individuals we took 3 photographs at the same day and location (of these we used only 1 image per individual in the main analyses). Assuming correct calibration of the photographs, color scores of individual images taken during the same day should contribute less to total variance in color scores (first component of the PCA) than the identity of the individual. We tested this by applying a random effects model for the analysis of variance components (Brown and Mosteller 1991) using Color Score as response and incorporating male identity (Male ID) and Photo Number as 2 random factors.
-
2)
We analyzed individual variation in facial hair coloration first by applying a 2-way nested analysis of variance followed by variance component analysis with Color Score as response and both Male ID and Patch (2 factor levels: left and right) as explanatory variables. Error structure reflected the nested design of repeated measures per individual. Owing to unbalanced numbers of individual images in 2006 we restricted this analysis to a data set comprising all images collected in 2007 (n 2007 = 63) for which all model assumptions were met and the error structure was normally distributed. To determine whether male facial color variation was related to group membership (groups A, B, F, J), age, social rank (dominant or subordinate), or reproductive success (infants per yr/male), we applied a linear mixed model (LMM) to the full data set of images collected in 2006 (n 2006 = 37) and 2007 (n 2007 = 63). Full model structure was: Response = Color Score; Fixed effects = Group, Age, Rank, Reproductive Success, and Year. Incorporating Male ID as random effect factor accounted for repeated measurements per individual male.
-
3)
To investigate temporal variation in facial coloration with regard to androgen level we first applied 2 LMMs to the total dataset exploring variability in male facial coloration and androgen levels with regard to short-term (≤4-mo study period, including the mating season) and long-term (between years) temporal changes using the following model structure: Response = Color Score or Androgen Level; Fixed effects = “Season,” Year; Random effect = Male ID. We further explored a direct relationship between androgen levels and male facial coloration via Spearman rank correlations on individual means of males that were part of the study population in both years. We used the variability in coloration of gray patches (n = 60; LMM: Response = Color Score gray; Fixed effect = Year; Random effect = Male ID) to control for androgen-dependent color change (Cotton et al. 2004).
We conducted all statistical analyses in R 2.8.1 (R Development Core Team 2008). We used the nlme-package (Pinheiro et al. 2008) to estimate LMM parameters with restricted maximum likelihood (REML) because we could not achieve model convergence with maximum likelihood (ML). We conducted model simplification by stepwise removal of nonsignificant parameters.
Results
Validation of the Color Assessment Method
Results of 120 regressions (3 color channels × 40 images) showed very high degrees of linearity in RGB values produced by the camera in response to a linear series of grays of known reflectance (r² range: 0.98–1, meanRed = 0.997 ± 0.005 SD, meanGreen = 0.996 ± 0.005, meanBlue = 0.996 ± 0.005, n = 120). In addition, the degree of RGB equalization was very high. The absolute value of the difference between the RGB-values in all seven grey squares was 0–16 (0.00–6.27% of a maximal possible difference of 255) and the mean ± SD difference from equality was 2.95 ± 2.59 (1.16%, n = 840). Gray values from calibrated images showed a close fit to required values.
Statistical validation of the hair coloration assessment method (variance component analysis) revealed that only 4% of the total variation in color score measurements was attributable to the error variability within a series of photos of 1 individual, whereas 96% was attributable to differences between individuals.
Individual Variation in Facial Hair Coloration
Standardized color scores of facial hair coloration ranged from −153.24 to 107.85 (mean = 0.00, SD = 57.78). A high color score indicated a rufous red color. Color scores differed significantly between individuals (F 12,126 = 14.13, p < 0.001; Fig. 2), but the location of the color measurement on the forehead (Patch) did not have a significant effect (F 1,126 = 0.01, p = 0.94). Male ID explained 97.6% of the overall variability whereas patch location contributed only 2.4% to the explanation of existing variability in the response variable. We collapsed levels of this factor in subsequent analyses.
Social groups (groups A, B, F, J; Fig. 2) did not differ systematically with regard to male facial coloration (t all_groups < 1.64, p all_groups > 0.20), and there were rufous and pale individuals in each social group. Individual differences in coloration between males were not related to age (t 1,63 = 0.41, p = 0.68; age range: 3–12 yr), dominance rank (t 1,63 = 1.05, p = 0.32) or reproductive success (t 1,63 = 1.20, p = 0.23; 0–3 infants per yr/male). Thus in this analysis, model simplification indicated best fit for a simple model with year as a covariate and Male ID as a random effect.
Temporal Variation in Facial Coloration with Regard to Androgen Levels
During both 4-mo study periods including the mating season, male facial color scores showed no consistent temporal variation (season: t 3,100 = −0.27, p = 0.80). However, color scores increased significantly between the years 2006 and 2007, with all males displaying consistently higher mean color scores in 2007 (year: t 1,100 = 12.23, p < 0.01, Fig. 3c). Color scores of gray control patches also varied between years (t 1,100 = 9.34, p < 0.01, Fig. 3b), but the effect size explaining the difference between gray patches between years was smaller than that explaining the difference between red patches between 2006 and 2007 (66.49 ± 6.79 SE vs. 98.89 ± 7.25 SE).
Individual variation in hair coloration and androgen level between years. Lines link repeated measures of the same individual in different years. a Color scores of color chart standard patches did not differ between years. b Color scores of gray control patches were significantly higher in 2007. c Color scores of red patches increased significantly between years, and effect size explaining the difference between red patches between years was greater than that explaining the difference between gray patches between years. d Androgen levels were significantly increased in 2007 versus 2006.
Fecal androgen levels increased significantly during the mating season in both years (t mating = 8.54, p < 0.001). Androgen levels were also significantly higher in 2007 than in 2006 across all individuals (t 1,98 = 6.12, p < 0.001; Fig. 3d), and levels in 2007 were also higher versus those measured in an earlier study in 1999 (Ostner et al. 2002). Correlation analyses revealed a positive relationship between individual androgen level and mean individual color score, and this relationship was significant in both years (p 2006 < 0.05, r² = 0.58, p 2007 < 0.05, r² = 0.59; Fig. 4).
Discussion
Measuring Primate Hair Coloration
An increased interest in the functional meaning of primate color paired with the use of digital photography as a method for the quantification of animal color (as opposed to spectrophotometry; Zuk and Decruyenaere 1994) enhanced the number of studies on the proximate and ultimate function of coloration in primate males and females (Bergman and Beehner 2008; Higham et al. 2008; Setchell et al. 2006). However, the majority of studies have focused on measuring skin coloration, while studies of the functional significance and physiological correlates of primate hair coloration are underrepresented (Bradley and Mundy 2008; but see Sumner and Mollon 2003). We measured primate facial hair coloration digitally in free-ranging red-fronted lemurs and explored its functional significance. We found that 1) correcting for even minor deviations from optimal exposure, 2) including information from multidimensional color spaces, and 3) using repeated measurements per individual gave reliable results for quantifying facial hair coloration in free-ranging lemurs. Statistical validation confirmed this, and we therefore recommend our method for application in further studies of hair coloration.
Signaling Character of Facial Coloration
Digital color assessments confirmed our visual impression that individual forehead coloration in red-fronted lemurs varies significantly among males. Yet, in contrast to other studies investigating the signaling function of male skin coloration (Bergman and Beehner 2008; Gerald 2001; Setchell and Wickings 2005), we found no support for status-dependent male coloration with regard to age or rank. We do not know if females chose the brightest male, as Cooper and Hosey (2003) suggested, and preferences for a particular stimulus may not translate directly into mating decisions in a system wherein potential mates are associated year-round (Setchell and Kappeler 2003). Our results indicate that individual male reproductive success is not a function of male facial coloration. In addition, researchers have shown previously that rank, and consequently reproductive success (Kappeler and Port 2008; Wimmer and Kappeler 2002), are not related to differences in androgen levels in this species (Ostner et al. 2002). Thus, the outcome of reproductive competition appears to be influenced by complex traits beyond simple male ornaments. Color differences between individuals may be based on genetic differences, operating as an identity rather than a quality signal (Dale 2000; Dale et al. 2001). In support of this hypothesis, juvenile males strongly resemble their father in facial color intensity (D. Clough, pers. obs.).
Physiological Regulation of Facial Coloration
Baseline male androgen levels were positively correlated to individual color scores in both years, suggesting that, like skin coloration in other species (Setchell and Dixson 2001b; Wickings and Dixson 1992), forehead color in red-fronted lemur males is influenced by testosterone. However, we found no evidence that increases in androgen levels resulted in short-term variation in male facial hair coloration. Given existing reports of hair color change in manipulative studies (Asa et al. 2007; A. Yoder, pers. comm. cited in Barthold et al. 2008), this result was surprising, as we expected rapid changes in hair coloration to co-occur with the pronounced changes in androgen levels during the mating season. Our finding suggests that hair physiology in lemurs does not allow changes in pigmentation over short-term time scales, possibly due to cyclic melanogenesis (Slominski and Paus 1993). Because current knowledge of hair physiology is derived from studies on mice and humans, further research on primate melanogenesis is needed. Owing to our study design, we cannot exclude that facial coloration changes occurred in a time-delayed manner, as we were able to collect color data only ≤ 8 wk after the significant androgen increase during the mating season. Nevertheless, results of this study suggest that hair coloration does not provide reliable short-term cues about male condition.
In contrast, comparison between years revealed an increase in coloration in all males from 2006 to 2007. This color intensification was not obvious to the observer during field work, but a period of absence from the field between the 2 study periods prevented direct comparison. Color changes of the red forehead were accompanied by increases in androgen levels, and gray body color also intensified between years. However, the effect size for gray patches was smaller than for red patches, suggesting a greater condition dependence of red patches (Cotton et al. 2004).
Combining the information on androgen and coloration described in the preceding text, our results indicate that the regulating effect of androgens is less clear-cut for hair coloration than for skin coloration. A certain threshold level of androgens may be required to induce noticeable changes in hair coloration (Asa et al. 2007). Roulin et al. (2008) reported similar findings for barn owls (Tyto alba), in which glucocorticoids altered pheomelanin production in feathers only during prolonged periods of stress, but where baseline variability in hormone levels did not correlate with variability in color.
Although the present study suggested a positive association between androgen level and color score, unexplained variability between years still remained. Because hair coloration is under the control of multiple factors, we briefly discuss other possible influences. In an experimental study on house sparrows (Passer domesticus), Veiga and Puerta (1996) proposed that nutritional constraints determine the expression of not only carotinoid, but also of melanin-based coloration, based on diet quality and fat reserves. Consequently, differences in diet composition or body constitution of red-fronted lemurs may also influence the intensity of coloration; data on these variables were not available for our study. Similarly, male health, i.e., immune status, parasite infection intensities, and other physiological challenges may act on the expression of facial hair coloration. In subsequent analyses, we thus plan to analyze intestinal parasite infection patterns between years.
Adaptive Function of Facial Coloration
Our data suggest that male facial coloration conveys some information on male androgen status, which might contain subtle cues about male quality, e.g., with regard to immunocompetence (Folstad and Karter 1992). However, because males with the brightest colors did not necessarily have the highest reproductive success, this preliminary study using a small sample of males, but from several social groups, could not shed light on the possible adaptive function of this potential color ornament. A promising approach for future studies might include information about the visual system, as only some trichromatic red-fronted lemur females can perceive the red color signal (Bradley et al. 2008), whereas other (dichromatic) females do not possess the physiological abilities to do so (Tan and Li 1999). Unfortunately, we were not able to gather information on the genetic constitution of visual abilities of the females in our study population, but might include this in future studies.
Summary and Conclusions
Quantitative assessment of male facial hair coloration in red-fronted lemurs showed that males differ with regard to their individual coloration, but this color trait did not have the potential to provide reliable cues to short-term changes in male androgen levels. Intrinsic factors did not explain the conspicuous individual variability in male coloration, and mean individual androgen levels explained only part of the existing variability. However, our findings suggest that prolonged increases in androgen levels were reflected in changes in male forehead coloration. Although we cannot exclude other factors possibly affecting long-term color changes, facial hair coloration appears to be partially under proximate control of androgens and may provide a long-term quality signal. Information on further male quality traits as well as individual visual abilities of females should be considered in future experimental studies of female choice.
References
Andersson, M. (1994). Sexual selection. Princeton, NJ: Princeton University Press.
Asa, C. S., Porton, I. J., & Junge, R. (2007). Reproductive cycles and contraception of black lemurs (Eulemur macaco macaco) with depot medroxyprogesterone acetate during the breeding season. Zoo Biology, 26, 289–298.
Barthold, J., Fichtel, C., & Kappeler, P. M. (2008). What is it going to be? Pattern and potential function of natal coat change in sexually dichromatic redfronted lemurs (Eulemur fulvus rufus). American Journal of Physical Anthropology, 138, 1–10.
Bergman, T. J., & Beehner, J. C. (2008). A simple method for measuring colour in wild animals: Validation and use on chest patch colour in geladas (Theropithecus gelada). Biological Journal of the Linnean Society, 94, 231–240.
Bradley, B. J., Irwin, M., Kappeler, P. M., Moisson, P., & Morelli, T. L. (2008). & Pastorini, J. The proximate is key to the ultimate: Identifying the genetic bases of primate pigmentation. In Congress of the International Primatological Society XXII.
Bradley, B. J., & Mundy, N. I. (2008). The primate palette: The evolution of primate coloration. Evolutionary Anthropology, 17, 97–111.
Brown, C., & Mosteller, F. (1991). Components of variance. In D. C. Hoaglin, F. Mosteller & J. W. Tukey (Eds.), Fundamentals of explanatory analysis of variance (pp. 193–251). New York: John Wiley & Sons.
Buchholz, R. (1995). Female choice, parasite load and male ornamentation in wild turkeys. Animal Behaviour, 50, 929–943.
Castanet, J., & Ortonne, J.-P. (1996). Hair melanin and hair color. In P. Jollès, H. Zahn & H. Höcker (Eds.), Formation and structure of human hair: Biology and structure (pp. 209–226). Basel: Birkhäuser.
Cooper, V. J., & Hosey, G. R. (2003). Sexual dichromatism and female preference in Eulemur fulvus subspecies. International Journal of Primatology, 24, 1177–1188.
Cotton, S., Fowler, K., & Pomiankowski, A. (2004). Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proceedings of the Royal Society B: Biological Sciences, 271, 771–783.
Dale, J. (2000). Ornamental plumage does not signal male quality in red-billed queleas. Proceedings of the Royal Society B: Biological Sciences, 267, 2143–2149.
Dale, J., Lank, D. B., & Reeve, H. K. (2001). Signaling individual identity versus quality: A model and case studies with ruffs, queleas, and house finches. American Naturalist, 158, 75–86.
Dixson, A. (1998). Primate sexuality. Oxford: Oxford University Press.
Endler, J. A. (1993). The color of light in forests and its implications. Ecological Monographs, 63, 1–27.
Folstad, I., & Karter, A. J. (1992). Parasites, bright males, and the immunocompetence handicap. American Naturalist, 139, 603–622.
Gerald, M. S. (2001). Primate colour predicts social status and aggressive outcome. Animal Behaviour, 61, 559–566.
Gerald, M. S. (2003). How color may guide the primate world: Possible relationships between sexual selection and sexual dichromatism. In C. B. Jones (Ed.), Sexual selection and reproductive competition in primates: New perspectives and directions (pp. 141–171). Norman: The American Society of Primatologists.
Gerald, M. S., Bernstein, J., Hinkson, R., & Fosbury, R. A. E. (2001). Formal method for objective assessment of primate colour. American Journal of Primatology, 53, 79–85.
Gerald, M. S., Waitt, C., Little, A. C., & Kraiselburd, E. (2007). Females pay attention to female secondary sexual color: An experimental study in Macaca mulatta. International Journal of Primatology, 28, 1–7.
Hamilton, W. D., & Zuk, M. (1982). Heritable true fitness and bright birds: A role for parasites? Science, 218, 384–387.
Hauser, M. (1996). The evolution of communication. Cambridge, MA: Bradford/MIT Press.
Hausfater, G., Gerhardt, H. C., & Klump, G. M. (1990). Parasites and mate choice in gray treefrogs, Hyla versicolor. American Naturalist, 30, 299–311.
Hearing, V. J. (1999). Biochemical control of melanogenesis and melanosomal organization. Journal of Investigative Dermatology Symposium Proceedings, 4, 24–28.
Higham, J. P., MacLarnon, A. M., Ross, C., Heistermann, M., & Semple, S. (2008). Baboon sexual swellings: Information content of size and color. Hormones and Behavior, 53, 452–462.
Hill, G. E. (1991). Plumage coloration is a sexually selected indicator of male quality. Nature, 350, 337–339.
Izard, K., Epps, B., & Simons, E. (1995). Reproduction in the brown lemur (Eulemur fulvus fulvus). American Journal of Primatology, 36, 129.
Jacobs, G. H. (1993). The distribution and nature of colour vision among the mammals. Biological Reviews of the Cambridge Philosophical Society, 68, 413–471.
Jacobs, G. H. (1999). Prospects for trichromatic color vision in male Cebus monkeys. Behavioural Brain Research, 101, 109–112.
Kappeler, P. M., & Port, M. (2008). Mutual tolerance or reproductive competition? Patterns of reproductive skew among male redfronted lemurs (Eulemur fulvus rufus). Behavioral Ecology and Sociobiology, 62, 1477–1488.
Kraus, C., Heistermann, M., & Kappeler, P. M. (1999). Physiological suppression of sexual function of subordinate males: A subtle form of intrasexual competition among male sifakas (Propithecus verreauxi)? Physiology & Behavior, 66, 855–861.
Loy, J. (1974). Changes in facial color associated with pregnancy in patas monkeys. Folia Primatolologica, 22, 251–257.
Maynard-Smith, J., & Harper, D. (2003). Animal signals. Oxford: Oxford University Press.
Milinski, M., & Bakker, T. C. M. (1990). Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature, 344, 330–333.
Mittermeier, R. A., Konstant, W. R., Hawkins, F., Louis, E. E., Langrand, O., Ratsimbazafy, J., et al. (2006). Lemurs of Madagascar. Washington, DC: Conservation International.
Ostner, J., & Kappeler, P. M. (1999). Central males instead of multiple pairs in redfronted lemurs, Eulemur fulvus rufus (Primates, Lemuridae)? Animal Behaviour, 58, 1069–1078.
Ostner, J., Kappeler, P. M., & Heistermann, M. (2002). Seasonal variation and social correlates of androgen excretion in male redfronted lemurs (Eulemur fulvus rufus). Behavioral Ecology and Sociobiology, 52, 485–495.
Ostner, J., Kappeler, P. M., & Heistermann, M. (2008). Androgen and glucocorticoid levels reflect seasonally occurring social challenges in male redfronted lemurs (Eulemur fulvus rufus). Behavioural Ecology and Sociobiology, 62, 627–638.
Palmer, A. E., London, W. T., Brown, R. L., & Rice, J. M. (1981). Color changes in the haircoat of patas monkeys (Erythrocebus patas). American Journal of Primatology, 1, 371–378.
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., & Team, R-Core. (2008). nlme: Linear and Nonlinear Mixed Effects Models. R-package version, 3, 1–89.
R Development Core Team. (2008). R: A language and environment for statistical computing, R Foundation for Statistical Computing. Vienna, Austria, http://www.R-project.org.
Randall, V. A. (2000). Androgens: The main regulator of human hair growth. In F. M. Camacho, V. A. Randall & V. H. Price (Eds.), Hair and its disorders. Biology, pathology and management (pp. 69–82). London: Martin Dunitz.
Randall, V. A. (2008). Androgens and hair growth. Dermatologic Therapy, 21, 314–328.
Ressel, S., & Schall, J. J. (1989). Parasites and showy males: malarial infection and color variation in fence lizards. Oecologica, 78, 158–164.
Rhodes, L., Argersinger, M. E., Gantert, L. T., Friscino, B. H., Hom, G., Pikounis, B., et al. (1997). Effects of administration of testosterone, dihydrotestosterone, oestrogen and fadrozole, an aromatase inhibitor, on sex skin colour in intact male rhesus macaques. Journal of Reproduction and Fertility, 111, 51–57.
Rohwer, S., & Ewald, P. W. (1981). The cost of dominance and advantage of subordination in a badge signaling system. Evolution, 35, 441–454.
Roulin, A., Almasi, B., Rossi-Pedruzzi, A., Ducrest, A.-L., Wakamatsu, K., Miksik, I., et al. (2008). Corticosterone mediates the condition-dependent component of melanin-based coloration. Animal Behaviour, 75, 1351–1358.
Setchell, J., & Dixson, A. (2001a). Changes in the secondary sexual adornments of male mandrills (Mandrillus sphinx) are associated with gain and loss of alpha status. Hormones and Behavior, 39, 177–184.
Setchell, J. M. (2005). Do female mandrills prefer brightly colored males? International Journal of Primatology, 26, 715–735.
Setchell, J. M., & Dixson, A. F. (2001b). Circannual changes in the secondary sexual adornments of semifree-ranging male and female mandrills (Mandrillus sphinx). American Journal of Primatology, 53, 109–121.
Setchell, J. M., & Kappeler, P. M. (2003). Selection in relation to sex in primates. Advances in the Study of Behaviour, 33, 87–174.
Setchell, J. M., Smith, T., Wickings, E. J., & Knapp, L. A. (2008). Social correlates of testosterone and ornamentation in male mandrills. Hormones and Behavior, 54, 365–372.
Setchell, J. M., & Wickings, E. (2005). Dominance, status signals and coloration in male mandrills (Mandrillus sphinx). Ethology, 111, 25–50.
Setchell, J. M., Wickings, E. J., & Knapp, L. A. (2006). Signal content of red facial colouration in female mandrills (Mandrillus sphinx). Proceedings of the Royal Society B: Biological Sciences, 273, 2395–2400.
Slominski, A., & Paus, R. (1993). Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. Journal of Investigative Dermatology, 101, 90S–97S.
Slominski, A., Tobin, D. J., Shibahara, S., & Wortsman, J. (2004a). Melanin pigmentation in mammalian skin and its hormonal regulation. Physiological Reviews, 84, 1155–1228.
Slominski, A., Wortsman, J., Plonka, P. M., Schallreuter, K. U., Paus, R., & Tobin, D. J. (2004b). Hair follicle pigmentation. Journal of Investigative Dermatology, 124, 13–21.
Stevens, M., Párraga, C. A., Cuthill, I. C., Partridge, J. C., & Troscianko, T. S. (2007). Using digital photography to study animal coloration. Biological Journal of the Linnean Society, 90, 211–237.
Sumner, P., & Mollon, J. D. (2003). Colors of primate pelage and skin: Objective assessment of conspicuousness. American Journal of Primatology, 59, 67–91.
Surridge, A. K., Osorio, D., & Mundy, N. I. (2003). Evolution and selection of trichromatic vision in primates. Trends in Ecology & Evolution, 18, 198–205.
Tan, Y., & Li, W.-H. (1999). Trichromatic vision in prosimians. Nature, 402, 36.
Vandenbergh, J. G. (1965). Hormonal basis of sex skin in male rhesus monkeys. General and Comparative Endocrinology, 5, 31–34.
Veiga, J. P., & Puerta, M. (1996). Nutritional constraints determine the expression of a sexual trait in the house sparrow, Passer domesticus. Proceedings of the Royal Society B: Biological Sciences, 263, 229–234.
von Engelhardt, N., Kappeler, P. M., & Heistermann, M. (2000). Androgen levels and female social dominance in Lemur catta. Proceedings of the Royal Society B: Biological Sciences, 267, 1533–1539.
Waitt, C., Little, A. C., Wolfensohn, S., Honess, P., Brown, A. P., Buchanan-Smith, H. M., et al. (2003). Evidence from rhesus macaques suggests that male coloration plays a role in female primate mate choice. Proceedings of the Royal Society B: Biological Sciences (Supplement), 270, S144–S146.
Wedekind, C. (1992). Detailed information about parasites revealed by sexual ornamentation. Proceedings of the Royal Society B: Biological Sciences, 247, 169–174.
West, P. M., & Packer, C. (2002). Sexual Selection, temperature, and the lion's mane. Science, 297, 1339–1343.
Wickings, E. J., & Dixson, A. F. (1992). Testicular function, secondary sexual development, and social status in male mandrills. Physiology & Behavior, 52, 909–916.
Wimmer, B., & Kappeler, P. M. (2002). The effects of sexual selection and life history on the genetic structure of redfronted lemur, Eulemur fulvus rufus, groups. Animal Behaviour, 64, 557–568.
Zahavi, A. (1975). Mate selection—a selection for a handicap. Journal of Theoretical Biology, 53, 205–214.
Ziegler, T., Hodges, K., Winkler, P., & Heistermann, M. (2000). Hormonal correlates of reproductive seasonality in wild female Hanuman langurs (Presbytis entellus). American Journal of Primatology, 51, 119–134.
Zuk, M., & Decruyenaere, J. (1994). Measuring individual variation in colour: A comparison of two techniques. Biological Journal of the Linnean Society, 53, 165–173.
Acknowledgments
We thank the Malagasy Ministère de l’ Environment et des Eaux et Forêts, the Department Biologie Animale de l’Université d’Antanarivo, and the Centre National de Formation, d’Etudes et de Recherche en Environnement et Foresterie de Morondava for authorizing and supporting research in Kirindy. We thank our field assistant Remi Ampataka for enormous assistance in collection of fecal samples; Julia Ostner for providing data on androgen levels in 1999 and Markus Port for providing paternity data, respectively; and Andrea Heistermann and Christina Oberdieck for valuable support in the laboratory. We thank Manfred Eberle and Bernhard Fink for helpful discussion on color measurements and James Higham, Joanna Setchell, and 2 anonymous reviewers for commenting on an earlier version of the manuscript. D. Clough’s attendance at IPS 2008 was funded by the British Ecological Society. D. Clough was supported by a Villigst e.V. graduate scholarship with additional funding from the Departments of Behavioral Ecology and Reproductive Biology, German Primate Center.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Clough, D., Heistermann, M. & Kappeler, P.M. Individual Facial Coloration in Male Eulemur fulvus rufus: A Condition-dependent Ornament?. Int J Primatol 30, 859–875 (2009). https://doi.org/10.1007/s10764-009-9379-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-009-9379-5






 , B:
, B:  ,F:
,F:  , J:
, J:  ). Data for 2007.
). Data for 2007.
