Abstract
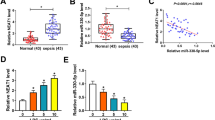
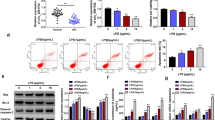
The lncRNA nuclear enriched abundant transcript 1 (NEAT1) promotes sepsis-inflammatory responses and acute kidney injury (AKI), but little known about the underlying mechanisms. This study aims to investigate the roles of NEAT1 in regulating macrophage polarization and its potential for alleviating inflammatory responses during sepsis pathogenesis. Mouse RAW264.7 macrophages were treated with lipopolysaccharide (LPS) as a cellular inflammatory model. NEAT1 shRNA, miR-125a-5p mimics, and TRAF6-overexpressing vector were used to transfect RAW264.7 cells. NEAT1, miR-125a-5p, and mRNA levels of functional genes were detected by quantitative RT-PCR. Protein abundances were analyzed by western blotting. Macrophage polarization was evaluated by flow cytometry. The bindings of miR-125a-5p with NEAT1 or TRAF6 gene were validated by dual luciferase reporter assay. LPS treatment promoted NEAT1 and suppressed miR-125a-5p expression in mouse macrophage cells. NEAT1 silencing by shRNAs promoted macrophage M2 polarization under LPS treatment, which upregulated miR-125a-5p expression, repressed TRAF6 expression and TAK1 protein phosphorylation in macrophages. These cellular and molecular changes induced by NEAT1 shRNAs were abrogated by miR-125a-5p inhibitors. Moreover, miR-125a-5p mimics suppressed TRAF6 expression and TAK1 protein phosphorylation in LPS-treated macrophages, thus causing macrophage M2 polarization under LPS treatment. TRAF6 overexpression abrogated the miR-125a-5p mimics-induced macrophage M2 polarization. miR-125a-5p could directly bind to NEAT1 or TRAF6 gene in macrophages. lncRNA NEAT1 knockdown ameliorates LPS-induced inflammation by promoting macrophage M2 polarization via miR-125a-5p/TRAF6/TAK1 axis.







Similar content being viewed by others
Abbreviations
- AAM:
-
Alternatively activated macrophage
- AKI:
-
Acute kidney injury
- AMPK:
-
AMP-activated protein kinase
- Arg-1:
-
Arginase-1
- ATAD2:
-
ATPase family AAA domain-containing protein 2
- ceRNA:
-
Competing endogenous RNA
- E2F3:
-
E2F transcription factor 3
- iNOS:
-
Inducible nitric oxide synthase
- IL-6:
-
Interleukin-6;
- KLF13:
-
Krueppel-like factor 13
- lncRNAs:
-
Long non-coding RNAs
- LPS:
-
Lipopolysaccharide
- miRNAs:
-
MicroRNAs
- NEAT1:
-
Nuclear enriched abundant transcript 1
- NF-κB:
-
Nuclear factor-kappa B
- PBMCs:
-
Peripheral blood mononuclear cells
- TAK1:
-
Transforming growth factor-activated kinase 1
- TRAF6:
-
Tumor necrosis factor receptor–associated factor 6
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor-alpha
References
Gotts, J.E., and M.A. Matthay. 2016. Sepsis: pathophysiology and clinical management. Bmj 353: i1585.
Gyawali, B., K. Ramakrishna, and A.S. Dhamoon. 2019. Sepsis: the evolution in definition, pathophysiology, and management. SAGE open medicine 7: 2050312119835043. https://doi.org/10.1177/2050312119835043.
Hotchkiss, R.S., G. Monneret, and D. Payen. 2013. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nature Reviews Immunology 13: 862–874.
Venet, F., and G. Monneret. 2017. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol. https://doi.org/10.1038/nrneph.2017.165.
Shakoory, B., J.A. Carcillo, W.W. Chatham, R.L. Amdur, H. Zhao, C.A. Dinarello, R.Q. Cron, and S.M. Opal. 2016. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Critical Care Medicine 44: 275–281. https://doi.org/10.1097/ccm.0000000000001402.
Hume, D. A. The Many Alternative faces of macrophage activation. Frontiers in Immunology 6, 370, doi:https://doi.org/10.3389/fimmu.2015.00370 (2015).
Kumar, V. 2018. Targeting macrophage immunometabolism: dawn in the darkness of sepsis. International Immunopharmacology 58: 173–185. https://doi.org/10.1016/j.intimp.2018.03.005.
Qiu, P., Y. Liu, and J. Zhang. 2019. Review: the role and mechanisms of macrophage autophagy in sepsis. Inflammation 42: 6–19. https://doi.org/10.1007/s10753-018-0890-8.
Watanabe, N., Y. Suzuki, S. Inokuchi, and S. Inoue. 2016. Sepsis induces incomplete M2 phenotype polarization in peritoneal exudate cells in mice. Journal of Intensive Care 4: 6. https://doi.org/10.1186/s40560-015-0124-1.
Feng, L., P. Song, H. Zhou, A. Li, Y. Ma, X. Zhang, H. Liu, G. Xu, Y. Zhou, X. Wu, Y. Shen, Y. Sun, X. Wu, and Q. Xu. 2014. Pentamethoxyflavanone regulates macrophage polarization and ameliorates sepsis in mice. Biochemical Pharmacology 89: 109–118. https://doi.org/10.1016/j.bcp.2014.02.016.
Wang, K.C., and H.Y. Chang. 2011. Molecular mechanisms of long noncoding RNAs. Molecular Cell 43: 904–914.
Ahmed, A.S.I., K. Dong, J. Liu, T. Wen, L. Yu, F. Xu, X. Kang, I. Osman, G. Hu, K.M. Bunting, D. Crethers, H. Gao, W. Zhang, Y. Liu, K. Wen, G. Agarwal, T. Hirose, S. Nakagawa, A. Vazdarjanova, and J. Zhou. 2018. Long noncoding RNA NEAT1 (nuclear paraspeckle assembly transcript 1) is critical for phenotypic switching of vascular smooth muscle cells. Proceedings of the National Academy of Sciences of the United States of America 115: E8660–E8667. https://doi.org/10.1073/pnas.1803725115.
Chakravarty, D., et al. 2014. The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nature Communications 5: 5383. https://doi.org/10.1038/ncomms6383.
Zhang, Q., C.Y. Chen, V.S. Yedavalli, and K.T. Jeang. 2013. NEAT1 long noncoding RNA and paraspeckle bodies modulate HIV-1 posttranscriptional expression. mBio4: e00596–e00512. https://doi.org/10.1128/mBio.00596-12.
Huang, S., et al. 2017. Diagnostic value of the lncRNA NEAT1 in peripheral blood mononuclear cells of patients with sepsis. Disease Markers. https://doi.org/10.1155/2017/7962836.
Wang, L., J.W. Xia, Z.P. Ke, and B.H. Zhang. 2019. Blockade of NEAT1 represses inflammation response and lipid uptake via modulating miR-342-3p in human macrophages THP-1 cells. Journal of Cellular Physiology 234: 5319–5326. https://doi.org/10.1002/jcp.27340.
Chen, Y., J. Qiu, B. Chen, Y. Lin, Y. Chen, G. Xie, J. Qiu, H. Tong, and D. Jiang. 2018. Long non-coding RNA NEAT1 plays an important role in sepsis-induced acute kidney injury by targeting miR-204 and modulating the NF-kappa B pathway. International Immunopharmacology 59: 252–260. https://doi.org/10.1016/j.intimp.2018.03.023.
Cai, Z., Li, J., Zhuang, Q., Zhang, X. & Shen, J. MiR-125a-5p ameliorates monocrotaline-induced pulmonary arterial hypertension by targeting the TGF-β1 and IL-6/STAT3 signaling pathways. Experimental & Molecular Medicine50 (2018).
Nicoletta, P. et al. Human microRNA hsa-miR-125a-5p interferes with expression of hepatitis B virus surface antigen. Nucleic Acids Research, 12 (2011).
Banerjee, S., et al. 2013. miR-125a-5p regulates differential activation of macrophages and inflammation. Journal of Biological Chemistry288: 35428–35436.
Banerjee, S., H. Cui, N. Xie, Z. Tan, S. Yang, M. Icyuz, V.J. Thannickal, E. Abraham, and G. Liu. 2013. miR-125a-5p regulates differential activation of macrophages and inflammation. Journal of Biological Chemistry 288: 35428–35436.
Xia, X., J. Cui, H.Y. Wang, L. Zhu, S. Matsueda, Q. Wang, X. Yang, J. Hong, Z. Songyang, Z.J. Chen, and R.F. Wang. 2011. NLRX1 negatively regulates TLR-induced NF-κB signaling by targeting TRAF6 and IKK. Immunity 34: 843–853.
Gao, M., X. Wang, X. Zhang, T. Ha, H. Ma, L. Liu, J.H. Kalbfleisch, X. Gao, R.L. Kao, D.L. Williams, and C. Li. 2015. Attenuation of cardiac dysfunction in polymicrobial sepsis by microRNA-146a is mediated via targeting of IRAK1 and TRAF6 expression. Journal of Immunology 195: 672–682. https://doi.org/10.4049/jimmunol.1403155.
An, R., J. Feng, C. Xi, J. Xu, and L. Sun. 2018. miR-146a attenuates sepsis-induced myocardial dysfunction by suppressing IRAK1 and TRAF6 via targeting ErbB4 expression. Oxidative medicine and cellular longevity2018: 7163057. https://doi.org/10.1155/2018/7163057.
Oya, A., E. Katsuyama, M. Morita, Y. Sato, T. Kobayashi, K. Miyamoto, T. Nishiwaki, A. Funayama, Y. Fujita, T. Kobayashi, M. Matsumoto, M. Nakamura, A. Kanaji, and T. Miyamoto. 2018. Tumor necrosis factor receptor-associated factor 6 is required to inhibit foreign body giant cell formation and activate osteoclasts under inflammatory and infectious conditions. Journal of Bone & Mineral Metabolism 36: 679–690.
Sorrentino, A., N. Thakur, S. Grimsby, A. Marcusson, V. von Bulow, N. Schuster, S. Zhang, C.H. Heldin, and M. Landström. 2008. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nature Cell Biology 10: 1199–1207. https://doi.org/10.1038/ncb1780.
Gallot, Y.S., J. McMillan, G. Xiong, K.R. Bohnert, A.R. Straughn, B.G. Hill, and A. Kumar. 2017. Distinct roles of TRAF6 and TAK1 in the regulation of adipocyte survival, thermogenesis program, and high-fat diet-induced obesity. Oncotarget 8: 112565–112583.
Yamada, H., T. Umemoto, M. Kakei, S.I. Momomura, M. Kawakami, S.E. Ishikawa, and K. Hara. 2017. Eicosapentaenoic acid shows anti-inflammatory effect via GPR120 in 3T3-L1 adipocytes and attenuates adipose tissue inflammation in diet-induced obese mice. Nutrition & Metabolism (London) 14: 33. https://doi.org/10.1186/s12986-017-0188-0.
Wang, L., J.-W. Xia, Z.-P. Ke, and B.-H. Zhang. 2019. Blockade of NEAT1 represses inflammation response and lipid uptake via modulating miR-342-3p in human macrophages THP-1 cells. Journal of Cellular Physiology 234: 5319–5326. https://doi.org/10.1002/jcp.27340.
Ke, H., L. Zhao, X. Feng, H. Xu, L. Zou, Q. Yang, X. Su, L. Peng, and B. Jiao. 2016. NEAT1 is required for survival of breast cancer cells through FUS and miR-548. Gene Regulation & Systems Biology 10: 11–17.
Sun, W., X. Lan, H. Zhang, Z. Wang, W. Dong, L. He, T. Zhang, P. Zhang, J. Liu, and Y. Qin. 2018. NEAT1_2 functions as a competing endogenous RNA to regulate ATAD2 expression by sponging microRNA-106b-5p in papillary thyroid cancer. Cell Death & Disease 9: 380. https://doi.org/10.1038/s41419-018-0418-z.
Zhang, F., et al. 2016. Identification of the long noncoding RNA NEAT1 as a novel inflammatory regulator acting through MAPK pathway in human lupus. Journal of Autoimmunity 75: 96.
Ha, M., and V.N. Kim. 2014. Regulation of microRNA biogenesis. Nature Reviews Molecular Cell Biology 15: 509–524.
Luo, M., Q. Sun, H. Zhao, J. Tao, and D. Yan. 2019. Long noncoding RNA NEAT1 sponges miR-495-3p to enhance myocardial ischemia-reperfusion injury via MAPK6 activation. Journal of Cellular Physiology. https://doi.org/10.1002/jcp.28791.
Chen, J.X., X. Xu, and S. Zhang. 2019. Silence of long noncoding RNA NEAT1 exerts suppressive effects on immunity during sepsis by promoting MicroRNA-125-dependent MCEMP1 downregulation. IUBMB Life. https://doi.org/10.1002/iub.2033.
Acknowledgments
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PNG 22 kb)
Rights and permissions
About this article
Cite this article
Wang, W., Guo, ZH. Downregulation of lncRNA NEAT1 Ameliorates LPS-Induced Inflammatory Responses by Promoting Macrophage M2 Polarization via miR-125a-5p/TRAF6/TAK1 Axis. Inflammation 43, 1548–1560 (2020). https://doi.org/10.1007/s10753-020-01231-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01231-y