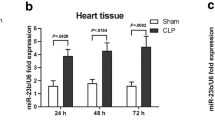
Abstract
Cardiomyopathy commonly occurs after sepsis and is closely associated with high mortality in clinic. Interferon regulatory factor-2 binding protein 2 (IRF2BP2) has been identified as a negative regulator of inflammation, but its role in septic cardiomyopathy is unknown. The current study aims to illuminate the regulatory function of IRF2BP2 on sepsis-induced cardiomyopathy and to explore the underlying mechanisms. Protein expression of IRF2BP2 in response to sepsis-induced cardiomyopathy was examined in the heart of mice challenged by LPS intraperitoneal injection. AAV9-delivered IRF2BP2 overexpression in the heart was applied to evaluate the regulatory role of IRF2BP2 in sepsis-induced myocardial depression, inflammatory response, and cell death. The molecular mechanisms underlying IRF2BP2-regulated cardiomyopathy were explored using western blot screening assay. Primary cardiomyocytes have been isolated to further confirm the role and mechanism of IRF2BP2 during septic cardiomyopathy. IRF2BP2 expression was dramatically increased in the heart of mice after LPS administration. AAV9-mediated IRF2BP2 overexpression significantly improved sepsis-induced cardiac dysfunction, inhibited inflammatory cell infiltration and cytokine production, and blocked cell death after LPS treatment. Mechanistically, IRF2BP2 activated AMPK signaling in cardiomyocytes, while inhibiting AMPK activation largely reversed IRF2BP2-benefited inflammatory suppression and cell survival. These findings clearly demonstrated that IRF2BP2 is a potent suppressor of sepsis-induced myocardial depression and related heart impairment. Targeting IRF2BP2 represents a promising therapeutic strategy for septic cardiomyopathy.








Similar content being viewed by others
Abbreviations
- IRF2BP2:
-
interferon regulatory factor-2 binding protein 2
- AAV:
-
adeno-associated virus
- LPS:
-
lipopolysaccharide
- CPC:
-
compound C
- LVEDd:
-
left ventricular end-diastolic diameter
- LVESd:
-
left ventricular end-systolic diameter
- EF:
-
ejection fraction
- FS:
-
fractional shortening
- RT-qPCR:
-
Real-time quantitative polymerase chain reaction
- TNF:
-
tumor necrosis factor
- ILs:
-
interleukins
- PVDF:
-
polyvinylidene fluoride
- SD:
-
standard deviation
- ANOVA:
-
analysis of variance
- NF-κB:
-
nuclear transcription factor kappa B
- P-P65:
-
phospho-nuclear transcription factor kappa B P65
- T-P65:
-
total nuclear transcription factor kappa B P65
- P-IκBα:
-
phospho-inhibitor of nuclear transcription factor kappa B α
- T-IκBα:
-
total inhibitor of nuclear transcription factor kappa B α
- Bcl2:
-
B cell lymphoma-2
- Bax:
-
BCL2-Associated X protein
- c-caspase3:
-
cleaved-caspase3
- P-AMPK:
-
phospho-adenosine monophosphate-activated protein kinase
- T-AMPK:
-
total-adenosine monophosphate-activated protein kinase
- P-ACC:
-
phospho-acetyl coenzyme A carboxylase
- T-ACC:
-
total-acetyl coenzyme A carboxylase
- P-mTOR:
-
phospho-mammalian target of rapamycin
- T-mTOR:
-
total-mammalian target of rapamycin
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
References
Fleischmann, C., A. Scherag, N.K. Adhikari, C.S. Hartog, T. Tsaganos, P. Schlattmann, D.C. Angus, K. Reinhart, and T. International Forum of Acute Care. 2016. Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. American Journal of Respiratory and Critical Care Medicine 193: 259–272.
Kakihana, Y., T. Ito, M. Nakahara, K. Yamaguchi, and T. Yasuda. 2016. Sepsis-induced myocardial dysfunction: Pathophysiology and management. Journal of Intensive Care 4: 22.
Neri, M., I. Riezzo, C. Pomara, S. Schiavone, and E. Turillazzi. 2016. Oxidative-nitrosative stress and myocardial dysfunctions in sepsis: Evidence from the literature and postmortem observations. Mediators of Inflammation 2016: 3423450.
Parrillo, J.E., M.M. Parker, C. Natanson, A.F. Suffredini, R.L. Danner, R.E. Cunnion, and F.P. Ognibene. 1990. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Annals of Internal Medicine 113: 227–242.
Clemente, G., A. Tuttolomondo, D. Colomba, R. Pecoraro, C. Renda, V. Della Corte, C. Maida, I. Simonetta, and A. Pinto. 2015. When sepsis affects the heart: A case report and literature review. World Journal of Clinical Cases 3: 743–750.
Remick, D.G. 2007. Pathophysiology of sepsis. The American Journal of Pathology 170: 1435–1444.
Abraham, E. 2003. Nuclear factor-kappaB and its role in sepsis-associated organ failure. The Journal of Infectious Diseases 187 (Suppl 2): S364–S369.
de Oliveira, S., E.E. Rosowski, and A. Huttenlocher. 2016. Neutrophil migration in infection and wound repair: Going forward in reverse. Nature Reviews. Immunology 16: 378–391.
Drifte, G., I. Dunn-Siegrist, P. Tissieres, and J. Pugin. 2013. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Critical Care Medicine 41: 820–832.
Beraud, A.S., C.V. Guillamet, J.L. Hammes, L. Meng, M.R. Nicolls, and J.L. Hsu. 2014. Efficacy of transthoracic echocardiography for diagnosing heart failure in septic shock. The American Journal of the Medical Sciences 347: 295–298.
Childs, K.S., and S. Goodbourn. 2003. Identification of novel co-repressor molecules for interferon regulatory factor-2. Nucleic Acids Research 31: 3016–3026.
Teng, A.C., D. Kuraitis, S.A. Deeke, A. Ahmadi, S.G. Dugan, B.L. Cheng, M.G. Crowson, P.G. Burgon, E.J. Suuronen, H.H. Chen, and A.F. Stewart. 2010. IRF2BP2 is a skeletal and cardiac muscle-enriched ischemia-inducible activator of VEGFA expression. The FASEB Journal 24: 4825–4834.
Ma, Y.L., J.L. Xia, and X. Gao. 2018. Suppressing Irf2bp2 expressions accelerates metabolic syndrome-associated brain injury and hepatic dyslipidemia. Biochemical and Biophysical Research Communications 503: 1651–1658.
Cruz, S.A., A. Hari, Z. Qin, P. Couture, H. Huang, D.C. Lagace, A.F.R. Stewart, and H.H. Chen. 2017. Loss of IRF2BP2 in microglia increases inflammation and functional deficits after focal ischemic brain injury. Frontiers in Cellular Neuroscience 11: 201.
Jovanovic, J.V., M.C. Chillon, C. Vincent-Fabert, R. Dillon, E. Voisset, N.C. Gutierrez, R.G. Sanz, A.A. Lopez, Y.G. Morgan, J. Lok, E. Solomon, E. Duprez, M.G. Diaz, and D. Grimwade. 2017. The cryptic IRF2BP2-RARA fusion transforms hematopoietic stem/progenitor cells and induces retinoid-sensitive acute promyelocytic leukemia. Leukemia 31: 747–751.
Keller, M.D., R. Pandey, D. Li, J. Glessner, L. Tian, S.E. Henrickson, I.K. Chinn, L. Monaco-Shawver, J. Heimall, C. Hou, F.G. Otieno, S. Jyonouchi, L. Calabrese, J. van Montfrans, J.S. Orange, and H. Hakonarson. 2016. Mutation in IRF2BP2 is responsible for a familial form of common variable immunodeficiency disorder. The Journal of Allergy and Clinical Immunology 138: 544–550 e544.
Chen, H.H., K. Keyhanian, X. Zhou, R.O. Vilmundarson, N.A. Almontashiri, S.A. Cruz, N.R. Pandey, N. Lerma Yap, T. Ho, C.A. Stewart, H. Huang, A. Hari, M. Geoffrion, R. McPherson, K.J. Rayner, and A.F. Stewart. 2015. IRF2BP2 reduces macrophage inflammation and susceptibility to atherosclerosis. Circulation Research 117: 671–683.
Zhang, H., and M.P. Reilly. 2015. IRF2BP2: A new player at the crossroads of inflammation and lipid metabolism. Circulation Research 117: 656–658.
Fang, J., T. Li, X. Zhu, K.Q. Deng, Y.X. Ji, C. Fang, X.J. Zhang, J.H. Guo, P. Zhang, H. Li, and X. Wei. 2017. Control of pathological cardiac hypertrophy by transcriptional corepressor IRF2BP2 (interferon regulatory factor-2 binding protein 2). Hypertension 70: 515–523.
Gude, N.A., G. Emmanuel, W. Wu, C.T. Cottage, K. Fischer, P. Quijada, J.A. Muraski, R. Alvarez, M. Rubio, E. Schaefer, and M.A. Sussman. 2008. Activation of notch-mediated protective signaling in the myocardium. Circulation Research 102: 1025–1035.
Wang, L., J. Wu, X. Guo, X. Huang, and Q. Huang. 2017 RAGE Plays a Role in LPS-Induced NF-kappaB activation and endothelial hyperpermeability. Sensors (Basel). 17(4): 722.
Li, T., Y. Su, X. Yu, D.S.A. Mouniir, J.F. Masau, X. Wei, and J. Yang. 2018. Trop2 guarantees cardioprotective effects of cortical bone-derived stem cells on myocardial ischemia/reperfusion injury. Cell Transplantation 27: 1256–1268.
Chen, L., Q. Li, L. Lei, and T. Li. 2018. Dioscin ameliorates cardiac hypertrophy through inhibition of the MAPK and Akt/GSK3beta/mTOR pathways. Life Sciences 209: 420–429.
Dombrovskiy, V.Y., A.A. Martin, J. Sunderram, and H.L. Paz. 2007. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Critical Care Medicine 35: 1244–1250.
Hochstadt, A., Y. Meroz, and G. Landesberg. 2011. Myocardial dysfunction in severe sepsis and septic shock: More questions than answers? Journal of Cardiothoracic and Vascular Anesthesia 25: 526–535.
Antonucci, E., E. Fiaccadori, K. Donadello, F.S. Taccone, F. Franchi, and S. Scolletta. 2014. Myocardial depression in sepsis: From pathogenesis to clinical manifestations and treatment. Journal of Critical Care 29: 500–511.
McGettrick, A.F., and L.A. O'Neill. 2013. How metabolism generates signals during innate immunity and inflammation. The Journal of Biological Chemistry 288: 22893–22898.
Qi, D., and L.H. Young. 2015. AMPK: Energy sensor and survival mechanism in the ischemic heart. Trends in Endocrinology and Metabolism 26: 422–429.
Clowes, G.H., Jr., M. Vucinic, and M.G. Weidner. 1966. Circulatory and metabolic alterations associated with survival or death in peritonitis: clinical analysis of 25 cases. Annals of Surgery 163: 866–885.
MacLean, L.D., W.G. Mulligan, A.P. McLean, and J.H. Duff. 1967. Patterns of septic shock in man--a detailed study of 56 patients. Annals of Surgery 166: 543–562.
Cordero, M.D., and B. Viollet. 2016. Editorial: AMPK: New frontiers in human diseases. Current Drug Targets 17: 852.
Li, X., J. Liu, Q. Lu, D. Ren, X. Sun, T. Rousselle, Y. Tan, and J. Li. 2018. AMPK: A therapeutic target of heart failure - not only metabolism regulation. Bioscience Reports.
O'Neill, L.A., and D.G. Hardie. 2013. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 493: 346–355.
Jeong, H.W., K.C. Hsu, J.W. Lee, M. Ham, J.Y. Huh, H.J. Shin, W.S. Kim, and J.B. Kim. 2009. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. American Journal of Physiology. Endocrinology and Metabolism 296: E955–E964.
Yang, Z., B.B. Kahn, H. Shi, and B.Z. Xue. 2010. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. The Journal of Biological Chemistry 285: 19051–19059.
Li, J., L. Zhong, F. Wang, and H. Zhu. 2017. Dissecting the role of AMP-activated protein kinase in human diseases. Acta Pharmaceutica Sinica B 7: 249–259.
Vaez, H., M. Rameshrad, M. Najafi, J. Barar, A. Barzegari, and A. Garjani. 2016. Cardioprotective effect of metformin in lipopolysaccharide-induced sepsis via suppression of toll-like receptor 4 (TLR4) in heart. European Journal of Pharmacology 772: 115–123.
Zhang, J., P. Zhao, N. Quan, L. Wang, X. Chen, C. Cates, T. Rousselle, and J. Li. 2017. The endotoxemia cardiac dysfunction is attenuated by AMPK/mTOR signaling pathway regulating autophagy. Biochemical and Biophysical Research Communications 492: 520–527.
Funding
This work was financially supported by the grant from National Natural Science Foundation of China (No. 81271348).
Author information
Authors and Affiliations
Contributions
Chengla Yi and Jie Xie contributed equally to this work as corresponding authors. Tianyu Li and Qiang Luo contributed equally to this work. Chengla Yi and Jie Xie conceived and designed all the experiments. Tianyu Li and Qiang Luo performed the experiments. Qiang Luo performed the revised experiments. Qiang Luo, Li He, Da Li, Qingnian Li, and Chuntao Wang analyzed the data and made the interpretation. Chengla Yi, Jie Xie, Tianyu Li, and Qiang Luo drafted and revised the article. All authors read and approved the final version.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Statement
All procedures involving animals were reviewed and approved by the Animal Care and Use Committee of Tongji Hospital of Huazhong University of Science and Technology and were performed according to the Declaration of Helsinki of the World Medical Association.The male C57BL6/J mice at the age of 10-12 weeks were applied in our experiments and were maintained in a standard specific-pathogen-free environment with 12-h light and 12-h dark cycle at the temperature of 20–26 °C. Food and water were accessed with ad libitum.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tianyu Li and Qiang Luo are co-first authors.
Rights and permissions
About this article
Cite this article
Li, T., Luo, Q., He, L. et al. Interferon Regulatory Factor-2 Binding Protein 2 Ameliorates Sepsis-Induced Cardiomyopathy via AMPK-Mediated Anti-Inflammation and Anti-Apoptosis. Inflammation 43, 1464–1475 (2020). https://doi.org/10.1007/s10753-020-01224-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01224-x