Abstract
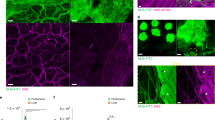
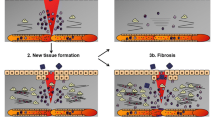
Extracellular matrix (ECM) proteins form the structural support for migration of leukocytes and provide multiple signals to assist in their functions during inflammatory conditions. Presence of pro-inflammatory mediators in the tissues results in the remodelling of matrices which could modify the functions of extravasated leukocytes. Previous reports have shown changes in the expression of ECM proteins during local inflammatory responses. In this study, we have investigated the time- and tissue-specific expression profile of key ECM proteins in systemic inflammation using lipopolysaccharide (LPS)-induced endotoxemia and cecal ligation and puncture (CLP) mouse models. The results show that compared to naïve tissues, within 12 h following CLP surgery, a 20–30-fold increase was observed in the expression of collagen-IV (Col-IV) transcripts in the mesentery tissues with a 2.4-fold increase in the protein by 24 h. However, Western blot band intensities indicated that vimentin and fibrinogen were remarkably expressed in more quantity compared to Col-IV. Secondly, in CLP group of mice, fibrinogen showed 6–40-fold increase in mRNA level in various tissues with about 2-fold increase in the protein level compared to respective naïve tissues. Similar studies in the LPS-injected mice showed up to 2–3 fold increase in the expression of Col-IV, fibrinogen and vimentin at protein level in the lungs. In such animals, although similar pattern was observed for fibrinogen in kidney and liver tissues, the mesentery showed prominent changes in Col-IV and vimentin mRNA compared to CLP. Further, bioinformatics analysis showed multiple pathways which could be associated with vimentin, Col-IV and fibrinogen under inflammatory conditions both in human and mouse. The current study will help in better understanding of possible signalling from ECM proteins in inflammatory microenvironment and may contribute in development of cell adhesion-based therapeutics.





Similar content being viewed by others
References
Kim, S.H., J. Turnbull, and S. Guimond. 2011. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. The Journal of Endocrinology 209: 139–151.
Bonnans, C., J. Chou, and Z. Werb. 2014. Remodelling the extracellular matrix in development and disease. Nature Reviews. Molecular Cell Biology 15: 786–801.
Overstreet, M.G., A. Gaylo, B.R. Angermann, A. Hughson, Y.M. Hyun, K. Lambert, M. Acharya, A.C. Billroth-Maclurg, A.F. Rosenberg, D.J. Topham, et al. 2013. Inflammation-induced interstitial migration of effector CD4(+) T cells is dependent on integrin alphaV. Nature Immunology 14: 949–958.
Shimshoni, E., D. Yablecovitch, L. Baram, I. Dotan, and I. Sagi. 2015. ECM remodelling in IBD: innocent bystander or partner in crime? The emerging role of extracellular molecular events in sustaining intestinal inflammation. Gut 64: 367–372.
Bhan, C., P. Dipankar, P. Chakraborty, and P.P. Sarangi. 2016. Role of cellular events in the pathophysiology of sepsis. Inflammation Research 65: 853–868.
Sarangi, P.P., Y.M. Hyun, Y.V. Lerman, A.P. Pietropaoli, and M. Kim. 2012. Role of beta1 integrin in tissue homing of neutrophils during sepsis. Shock 38: 281–287.
Rittirsch, D., M.A. Flierl, D.E. Day, B.A. Nadeau, S.R. McGuire, L.M. Hoesel, K. Ipaktchi, F.S. Zetoune, J.V. Sarma, L. Leng, et al. 2008. Acute lung injury induced by lipopolysaccharide is independent of complement activation. Journal of Immunology 180: 7664–7672.
Cox, T.R., and J.T. Erler. 2011. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Disease Models & Mechanisms 4: 165–178.
Kumawat, K., M.H. Menzen, I.S. Bos, H.A. Baarsma, P. Borger, M. Roth, M. Tamm, A.J. Halayko, M. Simoons, A. Prins, et al. 2013. Noncanonical WNT-5A signaling regulates TGF-beta-induced extracellular matrix production by airway smooth muscle cells. The FASEB Journal 27: 1631–1643.
Sarangi, P.P., H.-w. Lee, Y.V. Lerman, A. Trzeciak, E.J. Harrower, A.R. Rezaie, and M. Kim. 2017. Activated protein C attenuates severe inflammation by targeting VLA-3high neutrophil subpopulation in mice. The Journal of Immunology 199: 2930–2936.
Szklarczyk, D., A.L. Gable, D. Lyon, A. Junge, S. Wyder, J. Huerta-Cepas, M. Simonovic, N.T. Doncheva, J.H. Morris, and P. Bork. STRING v11. 2018. Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Research 47: D607–D613.
Wasserstein, R.L., A.L. Schirm, and N.A. Lazar. 2019. Moving to a world beyond “p< 0.05”. Taylor & Francis.
Lu, P., K. Takai, V.M. Weaver, and Z. Werb. 2011. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harbor Perspectives in Biology 3.
Lerman, Y.V., K. Lim, Y.M. Hyun, K.L. Falkner, H. Yang, A.P. Pietropaoli, A. Sonnenberg, P.P. Sarangi, and M. Kim. 2014. Sepsis lethality via exacerbated tissue infiltration and TLR-induced cytokine production by neutrophils is integrin α3β1-dependent. Blood. 124: 3515–3523.
Kuzmich, N., K. Sivak, V. Chubarev, Y. Porozov, T. Savateeva-Lyubimova, and F. Peri. 2017. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines 5: 34.
Gordon, A., A. Lagan, E. Aganna, L. Cheung, C. Peters, M. McDermott, J. Millo, K. Welsh, P. Holloway, and G. Hitman. 2004. TNF and TNFR polymorphisms in severe sepsis and septic shock: a prospective multicentre study. Genes and Immunity 5: 631–640.
Solovjov, D.A., E. Pluskota, and E.F. Plow. 2005. Distinct roles for the alpha and beta subunits in the functions of integrin alphaMbeta2. The Journal of Biological Chemistry 280: 1336–1345.
Kopec, A.K., N. Joshi, H. Cline-Fedewa, A.V. Wojcicki, J.L. Ray, B.P. Sullivan, J.E. Froehlich, B.F. Johnson, M.J. Flick, and J.P. Luyendyk. 2017. Fibrin(ogen) drives repair after acetaminophen-induced liver injury via leukocyte alphaMbeta2 integrin-dependent upregulation of Mmp12. Journal of Hepatology 66: 787–797.
Mariscal, I., and L.-B.G. Joanne. 2011. Fibrinogen, coagulation times and some inflammation markers in patients with different stages of. Am Soc Hematology: Sepsis.
Rittirsch, D., M.A. Flierl, and P.A. Ward. 2008. Harmful molecular mechanisms in sepsis. Nature Reviews. Immunology 8: 776–787.
Hyun, Y.M., C.T. Lefort, and M. Kim. 2009. Leukocyte integrins and their ligand interactions. Immunologic Research 45: 195–208.
Rittirsch, D., L.M. Hoesel, and P.A. Ward. 2007. The disconnect between animal models of sepsis and human sepsis. Journal of leukocyte biology. 81: 137–143.
Bekaert, S., M. Fillet, B. Detry, M. Pichavant, R. Maree, A. Noel, N. Rocks, and D. Cataldo. 2017. Inflammation-generated extracellular matrix fragments drive lung metastasis. Cancer Growth Metastasis 10: 1179064417745539.
Davalos, D., and K. Akassoglou. 2012. Fibrinogen as a key regulator of inflammation in disease. Seminars in Immunopathology 34: 43–62.
Simpson-Haidaris, P.J., M.A. Courtney, T.W. Wright, R. Goss, A. Harmsen, and F. Gigliotti. 1998. Induction of fibrinogen expression in the lung epithelium during Pneumocystis carinii pneumonia. Infection and Immunity 66: 4431–4439.
Nieminen, M., T. Henttinen, M. Merinen, F. Marttila-Ichihara, J.E. Eriksson, and S. Jalkanen. 2006. Vimentin function in lymphocyte adhesion and transcellular migration. Nature Cell Biology 8: 156–162.
dos Santos, G., M.R. Rogel, M.A. Baker, J.R. Troken, D. Urich, L. Morales-Nebreda, J.A. Sennello, M.A. Kutuzov, A. Sitikov, J.M. Davis, A.P. Lam, P. Cheresh, D. Kamp, D.K. Shumaker, G.R.S. Budinger, and K.M. Ridge. 2015. Vimentin regulates activation of the NLRP3 inflammasome. Nature Communications 6: 6574.
Kim, J., J. Jang, C. Yang, E.J. Kim, H. Jung, and C. Kim. 2016. Vimentin filament controls integrin alpha5beta1-mediated cell adhesion by binding to integrin through its Ser38 residue. FEBS Letters 590: 3517–3525.
Pipoly, D.J., and E.C. Crouch. 1987. Degradation of native type IV procollagen by human neutrophil elastase. Implications for leukocyte-mediated degradation of basement membranes. Biochemistry 26: 5748–5754.
Gaggar, A., and N. Weathington. 2016. Bioactive extracellular matrix fragments in lung health and disease. The Journal of Clinical Investigation 126: 3176–3184.
Iwabuchi, K., I. Nagaoka, A. Someya, and T. Yamashita. 1996. Type IV collagen-binding proteins of neutrophils: possible involvement of L-selectin in the neutrophil binding to type IV collagen. Blood 87: 365–372.
Funding
The Department of Biotechnology, Govt. of India (102/IFD/SAN/1671/2014-2015) to P.P.S., University Grants Commission, Govt. of India fellowship to CB (Sr. No-2061330682) and SPD (Sr. No-2061430670), Indian Council of Medical Research, Govt. of India fellowship to PD, and, Ministry of Human Resource Development, Govt. of India fellowship to PC (MHR 02-23-200-429) provided funding.
Author information
Authors and Affiliations
Contributions
PC, SD, CB and PD performed the in vivo experiments. CB and SD performed qRT-PCR and Western blot experiments and analysed the data. PD and PC assisted in RNA isolation from animal tissues and performing experiments. PK performed the bioinformatics studies and analysed the data. PPS conceived and directed the study. CB, SD and PPS wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 11681 kb)
Rights and permissions
About this article
Cite this article
Bhan, C., Dash, S.P., Dipankar, P. et al. Investigation of Extracellular Matrix Protein Expression Dynamics Using Murine Models of Systemic Inflammation. Inflammation 42, 2020–2031 (2019). https://doi.org/10.1007/s10753-019-01063-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01063-5