Abstract
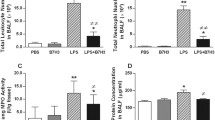
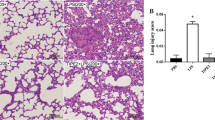
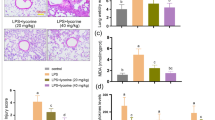
Acute lung injury (ALI)/acute respiratory distress syndrome is characterized by increased pulmonary inflammation, where T helper 17 (Th17) cells play an important regulatory role. Notch signaling critically regulates Th17 differentiation and is known to be linked with proximal T cell by protein kinase C theta (PKCθ). We hypothesized that PKCθ inhibition could attenuate ALI by suppressing Th17 response via the Notch signaling pathway. Male C57BL/6 mice were treated with phosphate-buffered saline (PBS), lipopolysaccharide (LPS), LPS and N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT, a Notch signaling inhibitor), or LPS and PKCθ inhibitor (PI), and the bronchoalveolar lavage fluid (BALF), blood, and lung tissues were harvested at 48 h after the LPS challenge. CD4+ T cells were treated with DAPT or PI and harvested after 72 h. PKCθ inhibition markedly attenuated pathological changes and decreased the wet to dry weight ratio of the mouse lungs. The total cell and neutrophil counts, tumor necrosis factor-α (TNF- α) in BALF, myeloperoxidase activity in lung tissue, and the leukocyte count in whole blood were markedly reduced by PKCθ inhibition. The concentration of interleukin (IL)-17 and IL-22 in BALF, and the percentage of CD4+IL-17A+ T cells in the lungs were significantly downregulated by PKCθ inhibition. A similar trend was observed for the expression of retinoic acid-related orphan receptor gamma t and IL-23 receptor after PKCθ inhibition accompanied with inactivation of the Notch signaling pathway in vivo and in vitro. Collectively, these data demonstrated that PKCθ inhibition protects against LPS-induced ALI by suppressing the differentiation and pathogenicity of Th17, at least partially, through a Notch-dependent mechanism.





Similar content being viewed by others
References
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD (2005) Incidence and outcomes of acute lung injury. The New England journal of medicine 353 (16):1685–1693.
Ferguson, N.D., E. Fan, L. Camporota, M. Antonelli, A. Anzueto, R. Beale, L. Brochard, R. Brower, A. Esteban, L. Gattinoni, A. Rhodes, A.S. Slutsky, J.L. Vincent, G.D. Rubenfeld, B.T. Thompson, and V.M. Ranieri. 2012. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Medicine 38 (10): 1573–1582.
Korn, T., E. Bettelli, M. Oukka, and V.K. Kuchroo. 2009. IL-17 and Th17 cells. Annual Review of Immunology 27: 485–517.
Williams, A.E., and R.C. Chambers. 2014. The mercurial nature of neutrophils: Still an enigma in ARDS? American Journal of Physiology. Lung Cellular and Molecular Physiology 306 (3): L217–L230.
Linden, A., H. Hoshino, and M. Laan. 2000. Airway neutrophils and interleukin-17. The European Respiratory Journal 15 (5): 973–977.
Glader, P., M.E. Smith, C. Malmhall, B. Balder, M. Sjostrand, I. Qvarfordt, and A. Linden. 2010. Interleukin-17-producing T-helper cells and related cytokines in human airways exposed to endotoxin. The European Respiratory Journal 36 (5): 1155–1164.
Ramezanpour, M., S. Moraitis, J.L. Smith, P.J. Wormald, and S. Vreugde. 2016. Th17 cytokines disrupt the airway mucosal barrier in chronic rhinosinusitis. Mediators of Inflammation 2016: 9798206.
Oppeltz, R.F., M. Rani, Q. Zhang, and M.G. Schwacha. 2011. Burn-induced alterations in toll-like receptor-mediated responses by bronchoalveolar lavage cells. Cytokine 55 (3): 396–401.
Li, J.T., A.C. Melton, G. Su, D.E. Hamm, M. LaFemina, J. Howard, X. Fang, S. Bhat, K.M. Huynh, C.M. O'Kane, R.J. Ingram, R.R. Muir, D.F. McAuley, M.A. Matthay, and D. Sheppard. 2015. Unexpected role for adaptive alphabetaTh17 cells in acute respiratory distress syndrome. Journal of Immunology (Baltimore, Md. : 1950) 195 (1): 87–95.
Yu, S., C. Liu, L. Li, T. Tian, M. Wang, Y. Hu, C. Yuan, L. Zhang, C. Ji, and D. Ma. 2015. Inactivation of Notch signaling reverses the Th17/Treg imbalance in cells from patients with immune thrombocytopenia. Laboratory Investigation; a Journal of Technical Methods and Pathology 95 (2): 157–167.
Keerthivasan, S., R. Suleiman, R. Lawlor, J. Roderick, T. Bates, L. Minter, J. Anguita, I. Juncadella, B.J. Nickoloff, I.C. Le Poole, L. Miele, and B.A. Osborne. 2011. Notch signaling regulates mouse and human Th17 differentiation. Journal of Immunology (Baltimore, Md. : 1950) 187 (2): 692–701.
Mukherjee, S., M.A. Schaller, R. Neupane, S.L. Kunkel, and N.W. Lukacs. 2009. Regulation of T cell activation by Notch ligand, DLL4, promotes IL-17 production and Rorc activation. Journal of Immunology (Baltimore, Md. : 1950) 182 (12): 7381–7388.
Piggott, K., J. Deng, K. Warrington, B. Younge, J.T. Kubo, M. Desai, J.J. Goronzy, and C.M. Weyand. 2011. Blocking the NOTCH pathway inhibits vascular inflammation in large-vessel vasculitis. Circulation 123 (3): 309–318.
Meyer Zu Horste, G., C. Wu, C. Wang, L. Cong, M. Pawlak, Y. Lee, W. Elyaman, S. Xiao, A. Regev, and V.K. Kuchroo. 2016. RBPJ controls development of pathogenic Th17 cells by regulating IL-23 receptor expression. Cell Reports 16 (2): 392–404.
Britton GJ, Ambler R, Clark DJ, Hill EV, Tunbridge HM, McNally KE, Burton BR, Butterweck P, Sabatos-Peyton C, Hampton-O'Neil LA, Verkade P, Wulfing Ch C, Wraith DC (2017) PKCtheta links proximal T cell and Notch signaling through localized regulation of the actin cytoskeleton. eLife 6. https://doi.org/10.7554/eLife.20003.
Zhang, E.Y., K.F. Kong, and A. Altman. 2013. The yin and yang of protein kinase C-theta (PKCtheta): A novel drug target for selective immunosuppression. Advances in Pharmacology (San Diego, Calif) 66: 267–312.
Kwon, M.J., R. Wang, J. Ma, and Z. Sun. 2010. PKC-theta is a drug target for prevention of T cell-mediated autoimmunity and allograft rejection. Endocrine, Metabolic & Immune Disorders Drug Targets 10 (4): 367–372.
Zhang, Q., Y. Zhao, M. Talukder, Y. Han, C. Zhang, X.N. Li, and J.L. Li. 2019. Di(2-ethylhexyl) phthalate induced hepatotoxicity in quail (Coturnix japonica) via modulating the mitochondrial unfolded protein response and NRF2 mediated antioxidant defense. The Science of the Total Environment 651 (Pt 1): 885–894.
Zhao, Y., J.H. Fan, Y. Luo, M. Talukder, X.N. Li, Y.Z. Zuo, and J.L. Li. 2019. Di-(2-ethylhexyl) phthalate (DEHP)-induced hepatotoxicity in quail (Coturnix japonica) via suppression of the heat shock response. Chemosphere 228: 685–693.
Qi, D., J. He, D. Wang, W. Deng, Y. Zhao, Y. Ye, and L. Feng. 2014. 17beta-estradiol suppresses lipopolysaccharide-induced acute lung injury through PI3K/Akt/SGK1 mediated up-regulation of epithelial sodium channel (ENaC) in vivo and in vitro. Respiratory Research 15: 159.
Luo, Y., X.N. Li, Y. Zhao, Z.H. Du, and J.L. Li. 2019. DEHP triggers cerebral mitochondrial dysfunction and oxidative stress in quail (Coturnix japonica) via modulating mitochondrial dynamics and biogenesis and activating Nrf2-mediated defense response. Chemosphere 224: 626–633.
Wang, H., X.N. Li, P.C. Li, W. Liu, Z.H. Du, and J.L. Li. 2019. Modulation of heat-shock response is associated with Di (2-ethylhexyl) phthalate (DEHP)-induced cardiotoxicity in quail (Coturnix japonica). Chemosphere 214: 812–820.
Meduri, G.U., D. Annane, G.P. Chrousos, P.E. Marik, and S.E. Sinclair. 2009. Activation and regulation of systemic inflammation in ARDS: Rationale for prolonged glucocorticoid therapy. Chest 136 (6): 1631–1643.
Adams, J.M., C.J. Hauser, D.H. Livingston, R.F. Lavery, Z. Fekete, and E.A. Deitch. 2001. Early trauma polymorphonuclear neutrophil responses to chemokines are associated with development of sepsis, pneumonia, and organ failure. The Journal of Trauma 51 (3): 452–456 discussion 456-457.
Geerts, L., P.G. Jorens, J. Willems, M. De Ley, and H. Slegers. 2001. Natural inhibitors of neutrophil function in acute respiratory distress syndrome. Critical Care Medicine 29 (10): 1920–1924.
Bertram, A., H. Zhang, S. von Vietinghoff, C. de Pablo, H. Haller, N. Shushakova, and K. Ley. 2012. Protein kinase C-theta is required for murine neutrophil recruitment and adhesion strengthening under flow. Journal of Immunology (Baltimore, Md. : 1950) 188 (8): 4043–4051.
Berger, C., J. Rossaint, H. Van Aken, M. Westphal, K. Hahnenkamp, and A. Zarbock. 2014. Lidocaine reduces neutrophil recruitment by abolishing chemokine-induced arrest and transendothelial migration in septic patients. Journal of Immunology (Baltimore, Md. : 1950) 192 (1): 367–376.
Skoutelis, A.T., V. Kaleridis, G.M. Athanassiou, K.I. Kokkinis, Y.F. Missirlis, and H.P. Bassaris. 2000. Neutrophil deformability in patients with sepsis, septic shock, and adult respiratory distress syndrome. Critical Care Medicine 28 (7): 2355–2359.
Yan, Z., Z. Xiaoyu, S. Zhixin, Q. Di, D. Xinyu, X. Jing, H. Jing, D. Wang, Z. Xi, Z. Chunrong, and W. Daoxin. 2016. Rapamycin attenuates acute lung injury induced by LPS through inhibition of Th17 cell proliferation in mice. Scientific Reports 6: 20156.
Lo Re, S., L. Dumoutier, I. Couillin, C. Van Vyve, Y. Yakoub, F. Uwambayinema, B. Marien, S. van den Brule, J. Van Snick, C. Uyttenhove, B. Ryffel, J.C. Renauld, D. Lison, and F. Huaux. 2010. IL-17A-producing gammadelta T and Th17 lymphocytes mediate lung inflammation but not fibrosis in experimental silicosis. Journal of Immunology (Baltimore, Md. : 1950) 184 (11): 6367–6377.
Koenders, M.I., L.A. Joosten, and W.B. van den Berg. 2006. Potential new targets in arthritis therapy: Interleukin (IL)-17 and its relation to tumour necrosis factor and IL-1 in experimental arthritis. Annals of the Rheumatic Diseases 65 (Suppl 3): iii29–iii33.
Wang, L., T. Yi, M. Kortylewski, D.M. Pardoll, D. Zeng, and H. Yu. 2009. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. The Journal of Experimental Medicine 206 (7): 1457–1464.
Park, H., Z. Li, X.O. Yang, S.H. Chang, R. Nurieva, Y.H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunology 6 (11): 1133–1141.
Miyamoto, M., O. Prause, M. Sjostrand, M. Laan, J. Lotvall, and A. Linden. 2003. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. Journal of Immunology (Baltimore, Md. : 1950) 170 (9): 4665–4672.
Sakaguchi, R., S. Chikuma, T. Shichita, R. Morita, T. Sekiya, W. Ouyang, T. Ueda, H. Seki, H. Morisaki, and A. Yoshimura. 2016. Innate-like function of memory Th17 cells for enhancing endotoxin-induced acute lung inflammation through IL-22. International Immunology 28 (5): 233–243.
Newcomb, D.C., J.Y. Cephus, M.G. Boswell, J.M. Fahrenholz, E.W. Langley, A.S. Feldman, W. Zhou, D.E. Dulek, K. Goleniewska, K.B. Woodward, C.M. Sevin, R.G. Hamilton, J.K. Kolls, and R.S. Peebles Jr. 2015. Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. The Journal of Allergy and Clinical Immunology 136 (4): 1025–1034.e1011.
Long, Q., Q. Luo, K. Wang, A. Bates, and A.K. Shetty. 2017. Mash1-dependent Notch signaling pathway regulates GABAergic neuron-like differentiation from bone marrow-derived mesenchymal stem cells. Aging and Disease 8 (3): 301–313.
Ivanov, I.I., B.S. McKenzie, L. Zhou, C.E. Tadokoro, A. Lepelley, J.J. Lafaille, D.J. Cua, and D.R. Littman. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126 (6): 1121–1133.
Kwon, M.J., J. Ma, Y. Ding, R. Wang, and Z. Sun. 2012. Protein kinase C-theta promotes Th17 differentiation via upregulation of Stat3. Journal of Immunology (Baltimore, Md. : 1950) 188 (12): 5887–5897.
Funding
This study was funded by the National Natural Science Foundation of China (Project number 81670071).
Author information
Authors and Affiliations
Contributions
Mengqin Li performed the experiments and wrote the manuscript. Yan Z, Jing He, Wang Deng, Li Cheng, and Zhi Jiang analyzed the data. Daoxin Wang designed the study and revised the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
All animal experiments were performed in accordance with the ethical guidelines of the National Institutes of Health on Animal Care, and the study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, M., Zhao, Y., He, J. et al. Protein Kinase C Theta Inhibition Attenuates Lipopolysaccharide-Induced Acute Lung Injury through Notch Signaling Pathway via Suppressing Th17 Cell Response in Mice. Inflammation 42, 1980–1989 (2019). https://doi.org/10.1007/s10753-019-01058-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01058-2