Abstract
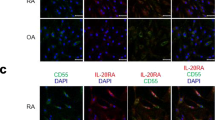
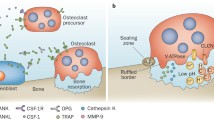
Osteoclast-associated receptor (OSCAR) is a co-stimulatory receptor in osteoclastogenesis. Synovial tissues from active rheumatoid arthritis (RA) patients express higher levels of OSCAR compared with osteoarthritic and normal patients; however, the comparison of OSCAR levels in different regions of active RA synovium has not been reported. The regulation of OSCAR by TNF-α and receptor activator of NF kappa β ligand (RANKL) in pre-osteoclasts/osteoclasts in vitro is unclear. OSCAR and tartrate-resistant acid phosphatase (TRAP) expression levels did not differ between the cartilage pannus junction (CPJ) and non-CPJ regions in active RA. We demonstrate a similar pattern of OSCAR expression in the CPJ and non-CPJ synovial tissue from patients with active RA. OSCAR was associated with mononuclear cells in both the lining and sub-lining and endothelial cells (von Willebrand factor positive). Pre-osteoclasts (TRAP-positive cells) were present in the lining and sub-lining of both regions. OSCAR messenger RNA (mRNA) expression and release by pre-oscteoclasts/osteoclasts was modulated by RANKL with/without TNF-α in vitro. Osteoclast resorption on dentine slices was significantly greater with TNF-α pre-treatment and RANKL (10 ng/ml) than RANKL 10 or 50 ng/ml alone or RANKL 10 ng/ml with TNF-α given from day 3 post-RANKL. The lower levels of OSCAR mRNA expression corresponded with high osteoclast activity levels.



Similar content being viewed by others
REFERENCES
Aletaha, D., T. Neogi, A. Silman, J. Funovits, D. Felson, C. Bingham, et al. 2010. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis and Rheumatism 62: 2569–2581.
van de Sande, M., M. de Hair, C. van der Leij, P. Klarenbeek, W. Bos, M. Smith, et al. 2011. Different stages of rheumatoid arthritis: features of the synovium in the preclinical phase. Annals of the Rheumatic Diseases 70: 772–777.
Ashton, B.A., I.K. Ashton, M.J. Marshall, and R.C. Butler. 1993. Localisation of vitronectin receptor immunoreactivity and tartrate resistant acid phosphatase activity in synovium from patients with inflammatory or degenerative arthritis. Annals of the Rheumatic Diseases 52: 133–137.
Chang, J.S., J.M. Quinn, A. Demaziere, C.J. Bulstrode, M.J. Francis, R.B. Duthie, et al. 1992. Bone resorption by cells isolated from rheumatoid synovium. Annals of the Rheumatic Diseases 51: 1223–1229.
Haynes, D., T. Crotti, M. Loric, G. Bain, G. Atkins, and D. Findlay. 2001. Osteoprotegerin and receptor activator of nuclear factor kappaB ligand (RANKL) regulate osteoclast formation by cells in the human rheumatoid arthritic joint. Rheumatology (Oxford, England) 40: 623–630.
Tsuboi, H., Y. Matsui, K. Hayashida, S. Yamane, M. Maeda-Tanimura, A. Nampei, et al. 2003. Tartrate resistant acid phosphatase (TRAP) positive cells in rheumatoid synovium may induce the destruction of articular cartilage. Annals of the Rheumatic Diseases 62: 196–203.
Gravallese, E.M., Y. Harada, J.T. Wang, A.H. Gorn, T.S. Thornhill, and S.R. Goldring. 1998. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. The American Journal of Pathology 152: 943–951.
Haynes, D., T. Crotti, H. Weedon, J. Slavotinek, V. Au, M. Coleman, et al. 2008. Modulation of RANKL and osteoprotegerin expression in synovial tissue from patients with rheumatoid arthritis in response to disease-modifying antirheumatic drug treatment and correlation with radiologic outcome. Arthritis and Rheumatism 59: 911–920.
Haynes, D.R., E. Barg, T.N. Crotti, C. Holding, H. Weedon, G.J. Atkins, et al. 2003. Osteoprotegerin expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathies and osteoarthritis and normal controls. Rheumatology 42: 123–134.
Takayanagi, H., H. Iizuka, T. Juji, T. Nakagawa, A. Yamamoto, T. Miyazaki, et al. 2000. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis and Rheumatism 43: 259–269.
Simonet, W., D. Lacey, C. Dunstan, M. Kelley, M. Chang, R. Lüthy, et al. 1997. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89: 309–319.
Udagawa, N., N. Takahashi, E. Jimi, K. Matsuzaki, T. Tsurukai, K. Itoh, et al. 1999. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand. Bone 25: 517–523.
Yasuda, H., N. Shima, N. Nakagawa, K. Yamaguchi, M. Kinosaki, S. Mochizuki, et al. 1998. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proceedings of the National Academy of Sciences of the United States of America 95: 3597–3602.
Zhao, B., M. Takami, Y. Miyamoto, T. Suzawa, A. Yamada, A. Mochizuki, et al. 2008. Characterization of synovial cell clones isolated from rheumatoid arthritis patients: possible involvement of TNF-alpha in reduction of osteoprotegerin in synovium. Cytokine 41: 61–70.
Kubota, A., K. Hasegawa, T. Suguro, and Y. Koshihara. 2004. Tumor necrosis factor-alpha promotes the expression of osteoprotegerin in rheumatoid synovial fibroblasts. The Journal of Rheumatology 31: 426–435.
Asagiri, M., K. Sato, T. Usami, S. Ochi, H. Nishina, H. Yoshida, et al. 2005. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. Journal of Experimental Medicine 202: 1261–1269.
Takayanagi, H., S. Kim, T. Koga, H. Nishina, M. Isshiki, H. Yoshida, et al. 2002. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Developmental Cell 3: 889–901.
Koga, T., M. Inui, K. Inoue, S. Kim, A. Suematsu, E. Kobayashi, et al. 2004. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428: 758–763.
Cella, M., C. Buonsanti, C. Strader, T. Kondo, A. Salmaggi, and M. Colonna. 2003. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. The Journal of Experimental Medicine 198: 645–651.
Kim, N., M. Takami, J. Rho, R. Josien, and Y. Choi. 2002. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. The Journal of Experimental Medicine 195: 201–209.
Ishikawa, S., N. Arase, T. Suenaga, Y. Saita, M. Noda, T. Kuriyama, et al. 2004. Involvement of FcRgamma in signal transduction of osteoclast-associated receptor (OSCAR). International Immunology 16: 1019–1025.
Kim, K., J.H. Kim, J. Lee, H.M. Jin, S.H. Lee, D.E. Fisher, et al. 2005. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. The Journal of Biological Chemistry 280: 35209–35216.
Crotti, Dharmapatni, Zannettino Alias, and Haynes Smith. 2012. The immunoreceptor tyrosine-based activation motif (ITAM)-related factors are increased in synovial tissue and vasculature of rheumatoid arthritic joints. Arthritis Research & Therapy 14: R245.
Herman, S., R. Müller, G. Krönke, J. Zwerina, K. Redlich, A. Hueber, et al. 2008. Induction of osteoclast-associated receptor, a key osteoclast costimulation molecule, in rheumatoid arthritis. Arthritis and Rheumatism 58: 3041–3050.
Zhao S, Guo Y, Ding N, Yang L, Zhang N. 2011. Changes in serum levels of soluble osteoclast-associated receptor in human rheumatoid arthritis. Chinese Medical Journal-Beijing.
Ndongo-Thiam, N., G. de Sallmard, J. Kastrup, and P. Miossec. 2014. Levels of soluble osteoclast-associated receptor (sOSCAR) in rheumatoid arthritis: link to disease severity and cardiovascular risk. Annals of the Rheumatic Diseases 73: 1276–1277.
Barrow, A., N. Raynal, T. Andersen, D. Slatter, D. Bihan, N. Pugh, et al. 2011. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. The Journal of Clinical Investigation 121: 3505–3516.
Goettsch, C., M. Rauner, K. Sinningen, S. Helas, N. Al-Fakhri, K. Nemeth, et al. 2011. The osteoclast-associated receptor (OSCAR) is a novel receptor regulated by oxidized low-density lipoprotein in human endothelial cells. Endocrinology 152: 4915–4926.
Schultz, H.S., L. Guo, P. Keller, A.J. Fleetwood, M. Sun, W. Guo, et al. 2016. OSCAR-collagen signaling in monocytes plays a proinflammatory role and may contribute to the pathogenesis of rheumatoid arthritis. European Journal of Immunology 46: 952–963.
Goettsch, C., S. Kliemt, K. Sinningen, M. von Bergen, L. Hofbauer, and S. Kalkhof. 2012. Quantitative proteomics reveals novel functions of osteoclast-associated receptor in STAT signaling and cell adhesion in human endothelial cells. Journal of Molecular and Cellular Cardiology 53: 829–837.
Starke, R., F. Ferraro, K. Paschalaki, N. Dryden, T. McKinnon, R. Sutton, et al. 2011. Endothelial von Willebrand factor regulates angiogenesis. Blood 117: 1071–1080.
Pendu, R., V. Terraube, O.D. Christophe, C.G. Gahmberg, P.G. de Groot, P.J. Lenting, et al. 2006. P-selectin glycoprotein ligand 1 and beta2-integrins cooperate in the adhesion of leukocytes to von Willebrand factor. Blood 108: 3746–3752.
Chu, C., M. Field, M. Feldmann, and R. Maini. 1991. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis and Rheumatism 34: 1125–1132.
Chu C, Field M, Allard S, Abney E. 1992 Detection of cytokines at the cartilage/pannus junction in patients with rheumatoid arthritis: implications for the role of cytokines in cartilage destruction and repair.
Barrow, A.D., Y. Palarasah, M. Bugatti, A.S. Holehouse, D.E. Byers, M.J. Holtzman, et al. 2015. OSCAR is a receptor for surfactant protein D that activates TNF-alpha release from human CCR2+ inflammatory monocytes. Journal of Immunology 194: 3317–3326.
Algate K, Haynes DR, Bartold PM, Crotti TN, Cantley MD. The effects of tumour necrosis factor-alpha on bone cells involved in periodontal alveolar bone loss; osteoclasts, osteoblasts and osteocytes. J Periodontal Res 2015.
Kobayashi, K., N. Takahashi, E. Jimi, N. Udagawa, M. Takami, S. Kotake, et al. 2000. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. The Journal of Experimental Medicine 191: 275–286.
Lam, J., S. Takeshita, J. Barker, O. Kanagawa, F. Ross, and S. Teitelbaum. 2000. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. The Journal of Clinical Investigation 106: 1481–1488.
Azuma, Y., K. Kaji, R. Katogi, S. Takeshita, and A. Kudo. 2000. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. The Journal of Biological Chemistry 275: 4858–4864.
MacRae, V.E., S.C. Wong, W. Smith, A. Gracie, I. McInnes, P. Galea, et al. 2007. Cytokine profiling and in vitro studies of murine bone growth using biological fluids from children with juvenile idiopathic arthritis. Clinical Endocrinology 67: 442–448.
Arnett, F.C., S.M. Edworthy, D.A. Bloch, D.J. Mcshane, J.F. Fries, N.S. Cooper, et al. 1988. The American-Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism 31: 315–324.
Burstone, M. 1958. Histochemical demonstration of acid phosphatases with naphthol AS-phosphates. Journal of the National Cancer Institute 21: 523–539.
Angel, N., N. Walsh, M. Forwood, M. Ostrowski, A. Cassady, and D. Hume. 2000. Transgenic mice overexpressing tartrate-resistant acid phosphatase exhibit an increased rate of bone turnover. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 15: 103–110.
Alias, E., A.S. Dharmapatni, A. Holding, G. Atkins, D. Findlay, D. Howie, et al. 2012. Polyethylene particles stimulate expression of ITAM-related molecules in peri-implant tissues and when stimulating osteoclastogenesis in vitro. Acta Biomaterialia 8: 3104–3112.
Kraan, M.C., J.J. Haringman, W.J. Post, J. Versendaal, F.C. Breedveld, and P.P. Tak. 1999. Immunohistological analysis of synovial tissue for differential diagnosis in early arthritis. Rheumatology (Oxford, England) 38: 1074–1080.
Holding, C.A., D.M. Findlay, R. Stamenkov, S.D. Neale, H. Lucas, A.S. Dharmapatni, et al. 2006. The correlation of RANK, RANKL and TNFalpha expression with bone loss volume and polyethylene wear debris around hip implants. Biomaterials 27: 5212–5219.
Livak, K.J., and T.D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25: 402–408.
ACKNOWLEDGEMENTS
This project was funded by the Allan and Beryl Stephens Grant from Arthritis Australia and an American Society for Bone Mineral Research (ASBMR) Grant in Aid Project Grant. We would like to thank Mrs. Helen Weedon from Rheumatology at the Repatriation General Hospital for providing patient information and histological preparation of the samples.
Author information
Authors and Affiliations
Contributions
K.A. and M.C. designed and K.A. performed the in vitro experiments with peripheral blood mononuclear cells including analysis of gene expression, TRAP staining and resorption. T.C., A.D. and R.C. designed and performed experiments with the clinical samples. M.W. and M.S. performed clinical analyses and interpretation. M.L. performed and directed statistical analyses. All authors contributed to the writing of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics approval was obtained from the Repatriation Hospital, Daw Park, SA (100911b) and the Southern Adelaide Clinical Human Research Ethics Committee (199.10) for synovial tissue collection. Ethics for obtaining human PBMCs from the Australian Red Cross Blood Service was through the University of Adelaide Human Ethics Committee (H-35-2001), in accordance with National Health and Medical Research Council of Australia guidelines. Informed written consent was obtained from all patients.
Conflict of Interest The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dharmapatni, A.A.S.S.K., Algate, K., Coleman, R. et al. Osteoclast-Associated Receptor (OSCAR) Distribution in the Synovial Tissues of Patients with Active RA and TNF-α and RANKL Regulation of Expression by Osteoclasts In Vitro . Inflammation 40, 1566–1575 (2017). https://doi.org/10.1007/s10753-017-0597-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0597-2