Abstract
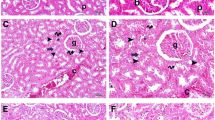
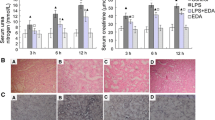
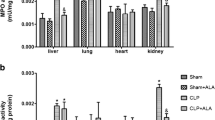
Oxidative stress causes mitochondrial impairment, the failure of energy production, and consequent organ dysfunctions. The aim of the present study was to investigate the potential therapeutic effects of mitochondrial antioxidant SS-31 on sepsis-induced organ dysfunctions and to explore the possible mechanism. Sepsis was induced by cecal ligation and puncture. Immediately and at 5 h after the operation, SS-31 (5 mg/kg) or vehicle was administered intraperitoneally. The levels of organ dysfunctions, malondialdehyde, superoxide dismutase, proinflammatory cytokines, pulmonary wet-to-dry weight ratio, myeloperoxidase activity, histological scores, nuclear factor kappa B p65, inducible nitric oxide synthase, reactive oxygen species, adenosine triphosphate, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells were assessed at the indicated time points. The 7-day survival rate was estimated by the Kaplan-Meier method. In the present study, SS-31 treatment significantly improved sepsis-induced organ dysfunctions as evidenced by decreased histological scores, increased arterial partial oxygen tension, and deceased serum alanine aminotransferase, urea nitrogen, and creatinine levels, which was accompanied by decreased levels of malondialdehyde, tumor necrosis factor-alpha, pulmonary myeloperoxidase activity, nuclear factor kappa B p65, inducible nitric oxide synthase, reactive oxygen species, and TUNEL-positive cells. In conclusion, our data suggested that the protective effects of SS-31 on sepsis-induced organ dysfunctions were associated with the inhibition of proinflammatory cytokines, oxidative stress, and apoptosis.








Similar content being viewed by others
References
Rubenfeld, G.D., E. Caldwell, E. Peabody, J. Weaver, D.P. Martin, M. Neff, E.J. Stern, and L.D. Hudson. 2005. Incidence and outcomes of acute lung injury. New England Journal of Medicine 353: 1685–1693.
Mouncey, P.R., T.M. Osborn, G.S. Power, D.A. Harrison, M.Z. Sadique, R.D. Grieve, R. Jahan, S.E. Harvey, D. Bell, J.F. Bion, T.J. Coats, M. Singer, J.D. Young, K.M. Rowan, and ProMISe Trial Investigators. 2015. Trial of early, goal-directed resuscitation for septic shock. New England Journal of Medicine 372: 1301–1311. doi:10.1056/NEJMoa1500896.
Ferrer, R., I. Martin-Loeches, G. Phillips, T.M. Osborn, S. Townsend, R.P. Dellinger, A. Artigas, C. Schorr, and M.M. Levy. 2014. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Critical Care Medicine 42: 1749–1755. doi:10.1097/CCM.0000000000000330.
Janz, D.R., J.A. Bastarache, T.W. Rice, G.R. Bernard, M.A. Warren, N. Wickersham, G. Sills, J.A. Oates, L.J. Roberts 2nd, and L.B. Ware. 2015. Acetaminophen for the reduction of oxidative injury in severe sepsis study group. Randomized, placebo-controlled trial of acetaminophen for the reduction of oxidative injury in severe sepsis: the acetaminophen for the reduction of oxidative injury in severe sepsis trial. Critical Care Medicine 43: 534–541. doi:10.1097/CCM.0000000000000718.
Lowes, D.A., N.R. Webster, M.P. Murphy, and H.F. Galley. 2013. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. British Journal of Anaesthesia 110: 472–480. doi:10.1093/bja/aes577.
Shen, H.H., K.K. Lam, P.Y. Cheng, C.W. Kung, S.Y. Chen, P.C. Lin, M.T. Chung, and Y.M. Lee. 2015. Alpha-lipoic acid prevents endotoxic shock and multiple organ dysfunction syndrome induced by endotoxemia in rats. Shock 43: 405–411. doi:10.1097/SHK.0000000000000295.
Ramsey, H., and M.X. Wu. 2014. Mitochondrial anti-oxidant protects IEX-1 deficient mice from organ dysfunction during endotoxemia. International Immunopharmacology 23: 658–663.
Singer, M. 2014. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 5: 66–72. doi:10.4161/viru.26907.
Wu, J., M. Zhang, S. Hao, M. Jia, M. Ji, L. Qiu, X. Sun, J. Yang, and K. Li. 2014. Mitochondria-targeted peptide reverses mitochondrial dysfunction and cognitive deficits in sepsis-associated encephalopathy. Molecular Neurobiology.
Birk, A.V., S. Liu, Y. Soong, W. Mills, P. Singh, J.D. Warren, S.V. Seshan, J.D. Pardee, and H.H. Szeto. 2013. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. Journal of the American Society of Nephrology 24: 1250–1261. doi:10.1681/ASN.2012121216.
Siegel, M.P., S.E. Kruse, J.M. Percival, J. Goh, C.C. White, H.C. Hopkins, T.J. Kavanagh, H.H. Szeto, P.S. Rabinovitch, and D.J. Marcinek. 2013. Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell 12: 763–771. doi:10.1111/acel.12102.
Thomas, D.A., C. Stauffer, K. Zhao, H. Yang, V.K. Sharma, H.H. Szeto, and M. Suthanthiran. 2007. Mitochondrial targeting with antioxidant peptide SS-31 prevents mitochondrial depolarization, reduces islet cell apoptosis, increases islet cell yield, and improves posttransplantation function. Journal of the American Society of Nephrology 18: 213–222.
Powers, S.K., M.B. Hudson, W.B. Nelson, E.E. Talbert, K. Min, H.H. Szeto, A.N. Kavazis, and A.J. Smuder. 2011. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Critical Care Medicine 39: 1749–1759. doi:10.1016/j.jacc.2010.12.044.
Dai, D.F., T. Chen, H. Szeto, M. Nieves-Cintrón, V. Kutyavin, L.F. Santana, and P.S. Rabinovitch. 2011. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. Journal of the American College of Cardiology 58: 73–82. doi:10.1016/j.jacc.2010.12.044.
Ji, M.H., G.M. Li, M. Jia, S.H. Zhu, D.P. Gao, Y.X. Fan, J. Wu, and J.J. Yang. 2013. Valproic acid attenuates lipopolysaccharide-induced acute lung injury in mice. Inflammation 36: 1453–1459. doi:10.1007/s10753-013-9686-z.
Li, G.M., M.H. Ji, X.J. Sun, Q.T. Zeng, M. Tian, Y.X. Fan, W.Y. Li, N. Li, and J.J. Yang. 2012. Effects of hydrogen-rich saline treatment on polymicrobial sepsis. Journal of Surgical Research 181: 279–286. doi:10.1016/j.jss.2012.06.058.
Ji, M., R. Li, G.M. Li, Y. Fan, L. Dong, J. Yang, Y.G. Peng, and J. Wu. 2012. Effects of combined levosimendan and vasopressin on pulmonary function in porcine septic shock. Inflammation 35(3): 871–880. doi:10.1007/s10753-011-9388-3.
Muravchick, S., and R.J. Levy. 2006. Clinical implications of mitochondrial dysfunction. Anesthesiology 105: 819–837.
Lorente, L., M.M. Martín, P. Abreu-González, A. Domínguez-Rodriguez, L. Labarta, C. Díaz, J. Solé-Violán, J. Ferreres, J. Cabrera, J.C. Igeño, and A. Jiménez. 2013. Sustained high serum malondialdehyde levels are associated with severity and mortality in septic patients. Critical Care 17: R290. doi:10.1186/cc13155.
Wang, H.W., W. Yang, J.Y. Lu, F. Li, J.Z. Sun, W. Zhang, N.N. Guo, L. Gao, and J.R. Kang. 2013. N-acetylcysteine administration is associated with reduced activation of NF-kB and preserves lung dendritic cells function in a zymosan-induced generalized inflammation model. Journal of Clinical Immunology l33: 649–660. doi:10.1007/s10875-012-9852-3.
Galvão, A.M., M.S. Wanderley, R.A. Silva, C.A. Filho, M.R. Melo-Junior, L.A. Silva, E.L. Streck, A.F. Dornelas de Andrade, M.B. Souza Maia, and C.M. Barbosa de Castro. 2014. Intratracheal co-administration of antioxidants and ceftriaxone reduces pulmonary injury and mortality rate in an experimental model of sepsis. Respirology 19: 1080–1087. doi:10.1111/resp.12363.
Ou, S.Y., H. Chu, P.W. Chao, S.M. Ou, Y.J. Lee, S.C. Kuo, S.Y. Li, C.J. Shih, and Y.T. Chen. 2014. Effect of the use of low and high potency statins and sepsis outcomes. Intensive Care Medicine 40: 1509–1517. doi:10.1007/s00134-014-3418-1.
Mertens, K., D.A. Lowes, N.R. Webster, J. Talib, L. Hall, M.J. Davies, J.H. Beattie, and H.F. Galley. 2015. Low zinc and selenium concentrations in sepsis are associated with oxidative damage and inflammation. British Journal of Anaesthesia 114: 990–999. doi:10.1093/bja/aev073.
Xie, Q.W., Y. Kashiwabara, and C. Nathan. 1994. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. Journal of Biological Chemistry 269: 4705–4708.
Zeng, M., W. He, L. Li, B. Li, L. Luo, X. Huang, K. Guan, and W. Chen. 2015. Ghrelin attenuates sepsis-associated acute lung injury oxidative stress in rats. Inflammation 38: 683–690. doi:10.1007/s10753-014-9977-z.
Lee, H.Y., M. Kaneki, J. Andreas, R.G. Tompkins, and J.A. Martyn. 2011. Novel mitochondria-targeted antioxidant peptide ameliorates burn-induced apoptosis and endoplasmic reticulum stress in the skeletal muscle of mice. Shock 36: 580–585. doi:10.1097/SHK.0b013e3182366872.
Guo, R., Y. Wang, A.W. Minto, R.J. Quigg, and P.N. Cunningham. 2004. Acute renal failure in endotoxemia is dependent on caspase activation. Journal of the American Society of Nephrology 15: 3093–3102.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 81271216, 81300946, 81400876); the grant from the Jinling Hospital (YYZD2014001); and the grant from Major Program of Public Health of Jiangsu Province (YG201409).
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Guoming Li and Jing Wu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, G., Wu, J., Li, R. et al. Protective Effects of Antioxidant Peptide SS-31 Against Multiple Organ Dysfunctions During Endotoxemia. Inflammation 39, 54–64 (2016). https://doi.org/10.1007/s10753-015-0222-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0222-1