ABSTRACT
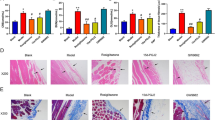
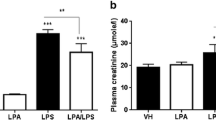
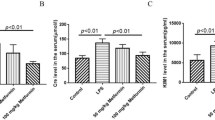
We assessed the anti-inflammatory effect of peroxisome proliferator-activated receptor (PPAR)-γ agonist, rosiglitazone, in a lipopolysaccharide (LPS)-induced peritonitis rat model. LPS was intraperitoneally injected into rats to establish peritonitis model. Male Sprague–Dawley (SD) rats were assigned to normal saline (the solvent of LPS), LPS, rosiglitazone plus LPS, and rosiglitazone alone. A simple peritoneal equilibrium test was performed with 20 ml 4.25 % peritoneal dialysis fluid. We measured the leukocyte count in dialysate and ultrafiltration volume. Peritoneal membrane histochemical staining was performed, and peritoneal thickness was assessed. CD40 and intercellular adhesion molecule-1 messenger RNA (ICAM-1 mRNA) levels in rat visceral peritoneum were detected by reverse transcription (RT)-PCR. IL-6 in rat peritoneal dialysis effluent was measured using enzyme-linked immunosorbent assay. The phosphorylation of NF-κB-p65 and IκBα was analyzed by Western blot. LPS administration resulted in increased peritoneal thickness and decreased ultrafiltration volume. Rosiglitazone pretreatment significantly decreased peritoneal thickness. In addition to CD40 and ICAM-1 mRNA expression, the IL-6, p-p65, and p-IκBα protein expressions were enhanced in LPS-administered animals. Rosiglitazone pretreatment significantly decreased ICAM-1 mRNA upregulation, secretion of IL-6 protein, and phosphorylation of NF-κB-p65 and IκBα without decreasing CD40 mRNA expression. Rosiglitazone has a protective effect in peritonitis, simultaneously decreasing NF-κB phosphorylation, suggesting that NF-κB signaling pathway mediated peritoneal inflammation induced by LPS. PPAR-γ might be considered a potential therapeutic target against peritonitis.





Similar content being viewed by others
REFERENCES
Krediet, R.T. 2007. 30 years of peritoneal dialysis development: the past and the future. Peritoneal Dialysis International 27: S35–S41.
Gokal, R. 2002. Peritoneal dialysis in the 21st century: an analysis of current problems and future developments. Journal of the American Society of Nephrology 13: S104–S116.
Fan, X., R. Huang, J. Wang, H. Ye, Q. Guo, C. Yi, J. Lin, Q. Zhou, F. Shao, X. Yu, and X. Yang. 2014. Risk factors for the first episode of peritonitis in Southern Chinese continuous ambulatory peritoneal dialysis patients. PLoS ONE 9, e107485.
Szeto, C.C., and K.M. Chow. 2007. Gram-negative peritonitis—the Achilles heel of peritoneal dialysis? Peritoneal Dialysis International 27: S267–S271.
Feng, X., X. Yang, C. Yi, Q. Guo, H. Mao, Z. Jiang, Z. Li, D. Chen, Y. Cui, and X. Yu. 2014. Escherichia coli peritonitis in peritoneal dialysis: the prevalence, antibiotic resistance and clinical outcomes in a South China dialysis center. Peritoneal Dialysis International 34: 308–16.
Devuyst, O., P.J. Margetts, and N. Topley. 2010. The pathophysiology of the peritoneal membrane. Journal of the American Society of Nephrology 21: 1077–1085.
Park, J.H., Y.G. Kim, M. Shaw, T.D. Kanneganti, Y. Fujimoto, K. Fukase, N. Inohara, and G. Núñez. 2007. Nod1/RICK and TLR signaling regulate chemokine and antimicrobial innate immune responses in mesothelial cells. Journal of Immunology 179: 514–521.
Laman, J.D., M. de Boer, and B.A. Hart. 1998. CD40 in clinical inflammation: from multiple sclerosis to atherosclerosis. Developmental Immunology 6: 215–222.
Zhang, Y.F., X. Yang, Y.J. Zhang, Y.L. Sun, X.L. Zou, Q.Y. Kong, X.Q. Dong, X.Q. Ye, and X.Q. Yu. 2006. Peroxisome proliferator-activated receptor-gamma is expressed by rat peritoneal mesothelial cells: its potential role in peritoneal cavity local defense. American Journal of Nephrology 26: 602–611.
Wu, J., X. Yang, Y.F. Zhang, S.F. Zhou, R. Zhang, X.Q. Dong, J.J. Fan, M. Liu, and X.Q. Yu. 2009. Angiotensin II upregulates Toll-like receptor 4 and enhances lipopolysaccharide-induced CD40 expression in rat peritoneal mesothelial cells. Inflammation Research 58: 473–482.
Forman, B.M., P. Tontonoz, J. Chen, R.P. Brun, B.M. Spiegelman, and R.M. Evans. 1995. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 83: 803–12.
Lehmann, J.M., L.B. Moore, T.A. Smith-Oliver, W.O. Wilkison, T.M. Willson, and S.A. Kliewer. 1995. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). Journal of Biological Chemistry 270: 12953–6.
Blanquart, C., O. Barbier, J.C. Fruchart, B. Staels, and C. Glineur. 2003. Peroxisome proliferator-activated receptors: regulation of transcriptional activities and roles in inflammation. Journal of Steroid Biochemistry and Molecular Biology 85: 267–73.
Liu, D., Z. Geng, W. Zhu, H. Wang, Y. Chen, and J. Liang. 2014. 15-deoxy-Δ12,14-prostaglandin J2 ameliorates endotoxin-induced acute lung injury in rats. Chinese Medical Journal 127: 815–20.
Kim, J.C., Y.H. Lee, M.K. Yu, N.H. Lee, J.D. Park, G. Bhattarai, and H.K. Yi. 2012. Anti-inflammatory mechanism of PPAR γ on LPS-induced pulp cells: role of the ROS removal activity. Archives of Oral Biology 57: 392–400.
Nizamutdinova, I.T., Y.M. Kim, H.J. Kim, H.G. Seo, J.H. Lee, and K.C. Chang. 2009. Carbon monoxide (from CORM-2) inhibits high glucose-induced ICAM-1 expression via AMP-activated protein kinase and PPAR-gamma activations in endothelial cells. Atherosclerosis 207: 405–11.
Sauter, M., K. Kastenmüller, F. Belling, M. Wörnle, R. Ladurner, T. Mussack, and T. Sitter. 2012. Activation of peroxisome proliferator-activated receptor-gamma by glitazones reduces the expression and release of monocyte chemoattractant protein-1 in human mesothelial cells. Mediators of Inflammation 2012: 217696.
Sandoval, P., J. Loureiro, G. González-Mateo, M.L. Pérez-Lozano, A. Maldonado-Rodríguez, J.A. Sánchez-Tomero, L. Mendoza, B. Santamaría, A. Ortiz, M. Ruíz-Ortega, R. Selgas, P. Martín, F. Sánchez-Madrid, A. Aguilera, and M. López-Cabrera. 2010. PPAR-γ agonist rosiglitazone protects peritoneal membrane from dialysis fluid-induced damage. Laboratory Investigation 90: 1517–32.
Sun, H., Y. Huang, X. Yu, Y. Li, J. Yang, R. Li, Y. Deng, and G. Zhao. 2008. Peroxisome proliferator-activated receptor gamma agonist, rosiglitazone, suppresses CD40 expression and attenuates inflammatory responses after lithium pilocarpine-induced status epilepticus in rats. International Journal of Developmental Neuroscience 26: 505–15.
Zhang, Y.J., X. Yang, Q.Y. Kong, Y.F. Zhang, W.Y. Chen, X.Q. Dong, X.Y. Li, and X.Q. Yu. 2006. Effect of 15d-PGJ2 on the expression of CD40 and RANTES induced by IFN-gamma and TNF-alpha on renal tubular epithelial cells (HK-2). American Journal of Nephrology 26: 356–62.
Kielian, T., M. McMahon, E.D. Bearden, A.C. Baldwin, P.D. Drew, and N. Esen. 2004. S. aureus-dependent microglial activation is selectively attenuated by the cyclopentenone prostaglandin 15-deoxy-delta12,14- prostaglandin J2 (15d-PGJ2). Journal of Neurochemistry 90: 1163–72.
Hontecillas, R., W.T. Horne, M. Climent, A.J. Guri, C. Evans, Y. Zhang, B.W. Sobral, and J. Bassaganya-Riera. 2011. Immunoregulatory mechanisms of macrophage PPAR-γ in mice with experimental inflammatory bowel disease. Mucosal Immunology 4: 304–13.
Luo, Y., C. Liang, C. Xu, Q. Jia, D. Huang, L. Chen, K. Wang, Z. Wu, and J. Ge. 2004. Ciglitazone inhibits oxidized-low density lipoprotein induced immune maturation of dendritic cells. Journal of Cardiovascular Pharmacology 44: 381–5.
Wakino, S., U. Kintscher, Z. Liu, S. Kim, F. Yin, M. Ohba, T. Kuroki, A.H. Schonthal, W.A. Hsueh, and R.E. Law. 2001. Peroxisome proliferator-activated receptor gamma ligands inhibit mitogenic induction of p21(Cip1) by modulating the protein kinase C delta pathway in vascular smooth muscle cells. Journal of Biological Chemistry 276: 47650–47657.
Gosset, P., A.S. Charbonnier, P. Delerive, J. Fontaine, B. Staels, J. Pestel, A.B.. Tonnel, and F. Trottein. 2001. Peroxisome proliferator-activated receptor gamma activators affect the maturation of human monocyte-derived dendritic cells. European Journal of Immunology 31(10): 2857–65.
Hayden, M.S., and S. Ghosh. 2008. Shared principles in NF-κB signaling. Cell 132: 344–362.
Yamamoto, Y., and R.B. Gaynor. 2004. IkappaB kinases: key regulators of the NF-kappaB pathway. Trends in Biochemical Sciences 29: 72–79.
Cruz, M.T., C.B. Duarte, M. Gonçalo, A.P. Carvalho, and M.C. Lopes. 2001. LPS induction of I kappa B-alpha degradation and iNOS expression in a skin dendritic cell line is prevented by the Janus kinase 2 inhibitor, tyrphostin b42. Nitric Oxide 5(1): 53–61.
Taggart, C.C., C.M. Greene, N.G. McElvaney, and S. O’Neill. 2002. Secretory leucoprotease inhibitor prevents lipopolysaccharide-induced IkappaBalpha degradation without affecting phosphorylation or ubiquitination. Journal of Biological Chemistry 277(37): 33648–53.
Verma, I.M., J.K. Stevenson, E.M. Schwarz, D. Van Antwerp, and S. Miyamoto. 1995. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes and Development 9(22): 2723–35.
Liu, Z.N., M. Zhao, Q. Zheng, H.Y. Zhao, W.J. Hou, and S.L. Bai. 2013. Inhibitory effects of rosiglitazone on paraquat-induced acute lung injury in rats. Acta Pharmacologica Sinica 34: 1317–24.
He, X., L. Feng, H. Meng, X. Wang, and S. Liu. 2012. Rosiglitazone protects dopaminergic neurons against lipopolysaccharide-induced neurotoxicity through inhibition of microglia activation. International Journal of Neuroscience 122: 532–40.
Leypoldt, J.K., C.D. Kemerath, and J.F. Gilson. 2007. Acute peritonitis in a C57BL/6 mouse model of peritoneal dialysis. Advances in Peritoneal Dialysis 23: 66–70.
ACKNOWLEDGMENTS
This work was funded by the Key Clinical Discipline Program of the Ministry of Health, China (Grant No. [2010]439), the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (Grant No. 2011BAI10B05), the Program of National Key Clinical Specialties, and Guangzhou Committee of Science and Technology, China (Grant No. 2010U1-E00831), and the program of Huadu district science and technology, Guangzhou, China (Grant No.13-HDWS2003).
Author Contributions
Prof Xiao YANG and Prof Xue-Qing YU conceived the study, participated in its design and coordination, and helped in drafting the manuscript. Dr. Yun-Fang ZHANG and Xun-Liang ZOU participated in study design, carried out the laboratory experiments, analyzed the data, interpreted the results, and wrote the manuscript. Jun WU carried out the laboratory experiments. All authors have approved the final version of the manuscript. We thank Ms. Xiu-Qing DONG and Dr Wen-Xing PENG for their technical advice and assistance.
Conflict of Interest
The authors declare that they have no competing interests.
Ethical approval
All experiments involving animals were approved by the Animal Care and Use Committee of the Sun Yat-sen University, Guangdong, China and carried out in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yun-Fang Zhang and Xun-Liang Zou contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, YF., Zou, XL., WU, J. et al. Rosiglitazone, a Peroxisome Proliferator-Activated Receptor (PPAR)-γ Agonist, Attenuates Inflammation Via NF-κB Inhibition in Lipopolysaccharide-Induced Peritonitis. Inflammation 38, 2105–2115 (2015). https://doi.org/10.1007/s10753-015-0193-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0193-2