Abstract
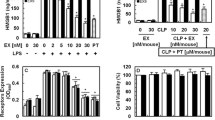
High mobility group box 1 (HMGB1) was recently shown to be an important extracellular mediator of severe vascular inflammatory disease, sepsis. Lysozyme protects us from the ever-present danger of bacterial infection and binds to bacterial lipopolysaccharide (LPS) with a high affinity. Here, we show, for the first time, the anti-septic effects of lysozyme in HMGB1-mediated inflammatory responses in vitro and in vivo. The data showed that lysozyme posttreatment suppressed LPS-mediated release of HMGB1 and HMGB1-mediated cytoskeletal rearrangement. Lysozyme also inhibited HMGB1-mediated hyperpermeability and leukocyte migration in human endothelial cells. In addition, lysozyme inhibited the HMGB1-mediated activation of Akt, nuclear factor-κB (NF-κB), extracellular regulated kinases (ERK) 1/2 and production of interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α), and chemoattractant protein-1 (MCP-1) in HUVECs. Furthermore, lysozyme reduced the cecal ligation and puncture (CLP)-induced release of HMGB1, migration of leukocytes, septic mortality, and pulmonary damage in mice. Collectively, these results suggest lysozyme as a candidate therapeutic agent for the treatment of vascular inflammatory diseases via inhibition of the HMGB1 signaling pathway.





Similar content being viewed by others
References
Russell, J.A., and K.R. Walley. 2013. Update in sepsis 2012. American Journal of Respiratory and Critical Care Medicine 187: 1303–1307.
Goodwin, G.H., C. Sanders, and E.W. Johns. 1973. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. European Journal of Biochemistry 38: 14–19.
Lotze, M.T., and K.J. Tracey. 2005. High-Mobility Group Box 1 Protein (HMGB1): Nuclear weapon in the immune arsenal. Nature Reviews. Immunology 5: 331–342.
Bae, J.S. 2012. Role of high mobility group box 1 in inflammatory disease: Focus on sepsis. Archives of Pharmacal Research 35: 1511–1523.
Hori, O., J. Brett, T. Slattery, et al. 1995. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. Journal of Biological Chemistry 270: 25752–25761.
Park, J.S., D. Svetkauskaite, Q. He, et al. 2004. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. Journal of Biological Chemistry 279: 7370–7377.
Bae, J.S., and A.R. Rezaie. 2011. Activated protein C inhibits high mobility group box 1 signaling in endothelial cells. Blood 118: 3952–3959.
Yang, H., M. Ochani, J. Li, et al. 2004. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proceedings of the National Academy of Sciences of the United States of America 101: 296–301.
Kelly, J.A., A.R. Sielecki, B.D. Sykes, M.N. James, and D.C. Phillips. 1979. X-ray crystallography of the binding of the bacterial cell wall trisaccharide NAM-NAG-NAM to lysozyme. Nature 282: 875–878.
Clingolani, R. 2014. Bioinspired approaches for human-centric technologies. New York: Springer.
Nakimbugwe, D., B. Masschalck, D. Deckers, L. Callewaert, A. Aertsen, and C.W. Michiels. 2006. Cell wall substrate specificity of six different lysozymes and lysozyme inhibitory activity of bacterial extracts. FEMS Microbiology Letters 259: 41–46.
Lee, W., S.K. Ku, and J.S. Bae. 2013. Emodin-6-O-beta-D-glucoside down-regulates endothelial protein C receptor shedding. Archives of Pharmacal Research 36: 1160–1165.
Bae, J.S., W. Lee, J.O. Nam, J.E. Kim, S.W. Kim, and I.S. Kim. 2014. Transforming growth factor beta-induced protein promotes severe vascular inflammatory responses. American Journal of Respiratory and Critical Care Medicine 189: 779–786.
Ku, S.K., M.S. Han, M.Y. Lee, Y.M. Lee, and J.S. Bae. 2014. Inhibitory effects of oroxylin A on endothelial protein C receptor shedding in vitro and in vivo. BMB Reports 47: 336–341.
Ku, S.K., and J.S. Bae. 2014. Antithrombotic activities of sulforaphane via inhibiting platelet aggregation and FIIa/FXa. Archives of Pharmacal Research 37: 1454–1463.
Ku, S.K., and J.S. Bae. 2014. Antiplatelet and antithrombotic activities of purpurogallin in vitro and in vivo. BMB Reports 47: 376–381.
Hofbauer, R., D. Moser, H. Salfinger, M. Frass, and S. Kapiotis. 1998. Sufentanil inhibits migration of human leukocytes through human endothelial cell monolayers. Anesthesia and Analgesia 87: 1181–1185.
Lee, W., H. Yoo, S.K. Ku, J.A. Kim, and J.S. Bae. 2013. Anticoagulant activities of piperlonguminine in vitro and in vivo. BMB Reports 46: 484–489.
Wang, H., H. Liao, M. Ochani, et al. 2004. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nature Medicine 10: 1216–1221.
Rittirsch, D., M.S. Huber-Lang, M.A. Flierl, and P.A. Ward. 2009. Immunodesign of experimental sepsis by cecal ligation and puncture. Nature Protocols 4: 31–36.
Lee, W., T.H. Kim, S.K. Ku, et al. 2012. Barrier protective effects of withaferin A in HMGB1-induced inflammatory responses in both cellular and animal models. Toxicology and Applied Pharmacology 262: 91–98.
Che, W., N. Lerner-Marmarosh, Q. Huang, et al. 2002. Insulin-like growth factor-1 enhances inflammatory responses in endothelial cells: Role of Gab1 and MEKK3 in TNF-alpha-induced c-Jun and NF-kappaB activation and adhesion molecule expression. Circulation Research 90: 1222–1230.
Bae, J.W., and J.S. Bae. 2011. Barrier protective effects of lycopene in human endothelial cells. Inflammation Research 60: 751–758.
Bae, J.S., L. Yang, C. Manithody, and A.R. Rezaie. 2007. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood 110: 3909–3916.
Bae, J.S., W. Lee, and A.R. Rezaie. 2012. Polyphosphate elicits proinflammatory responses that are counteracted by activated protein C in both cellular and animal models. Journal of Thrombosis and Haemostasis 10: 1145–1151.
Lee, W., S.K. Ku, J.A. Kim, T. Lee, and J.S. Bae. 2013. Inhibitory effects of epi-sesamin on HMGB1-induced vascular barrier disruptive responses in vitro and in vivo. Toxicology and Applied Pharmacology 267: 201–208.
Ozdulger, A., I. Cinel, O. Koksel, et al. 2003. The protective effect of N-acetylcysteine on apoptotic lung injury in cecal ligation and puncture-induced sepsis model. Shock 19: 366–372.
Mullins, G.E., J. Sunden-Cullberg, A.S. Johansson, et al. 2004. Activation of human umbilical vein endothelial cells leads to relocation and release of high-mobility group box chromosomal protein 1. Scandinavian Journal of Immunology 60: 566–573.
Buras, J.A., B. Holzmann, and M. Sitkovsky. 2005. Animal models of sepsis: Setting the stage. Nature Reviews Drug Discovery 4: 854–865.
Diehl, K.H., R. Hull, D. Morton, et al. 2001. A good practice guide to the administration of substances and removal of blood, including routes and volumes. Journal of Applied Toxicology 21: 15–23.
Sava, G., L. Perissin, S. Zorzet, and C. Callerio. 1986. Antineoplastic effects of egg-white lysozyme in mice bearing solid metastasizing tumors. Anticancer Research 6: 183–186.
Murakami, S., J. Nagai, K. Fujii, R. Yumoto, and M. Takano. 2008. Influences of dosage regimen and co-administration of low-molecular weight proteins and basic peptides on renal accumulation of arbekacin in mice. Journal of Antimicrobial Chemotherapy 61: 658–664.
Haverdings, R.F.G., M. Haas, A.R. Greupink, et al. 2001. Potentials and limitations of the low-molecular-weight protein lysozyme as a carrier for renal drug targeting. Renal Failure 23: 397–409.
Sama, A.E., J. D’Amore, M.F. Ward, G. Chen, and H. Wang. 2004. Bench to bedside: HMGB1-a novel proinflammatory cytokine and potential therapeutic target for septic patients in the emergency department. Academic Emergency Medicine 11: 867–873.
Berman, R.S., J.D. Frew, and W. Martin. 1993. Endotoxin-induced arterial endothelial barrier dysfunction assessed by an in vitro model. British Journal of Pharmacology 110: 1282–1284.
Goldblum, S.E., X. Ding, T.W. Brann, and J. Campbell-Washington. 1993. Bacterial lipopolysaccharide induces actin reorganization, intercellular gap formation, and endothelial barrier dysfunction in pulmonary vascular endothelial cells: Concurrent F-actin depolymerization and new actin synthesis. Journal of Cellular Physiology 157: 13–23.
Wolfson, R.K., E.T. Chiang, and J.G. Garcia. 2011. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvascular Research 81: 189–197.
Yang, H., H. Wang, C.J. Czura, and K.J. Tracey. 2005. The cytokine activity of HMGB1. Journal of Leukocyte Biology 78: 1–8.
Qin, Y.H., S.M. Dai, G.S. Tang, et al. 2009. HMGB1 enhances the proinflammatory activity of lipopolysaccharide by promoting the phosphorylation of MAPK p38 through receptor for advanced glycation end products. Journal of Immunology 183: 6244–6250.
Sun, C., C. Liang, Y. Ren, et al. 2009. Advanced glycation end products depress function of endothelial progenitor cells via p38 and ERK 1/2 mitogen-activated protein kinase pathways. Basic Research in Cardiology 104: 42–49.
Schnittler, H.J., S.W. Schneider, H. Raifer, et al. 2001. Role of actin filaments in endothelial cell-cell adhesion and membrane stability under fluid shear stress. Pflügers Archiv 442: 675–687.
Friedl, J., M. Puhlmann, D.L. Bartlett, et al. 2002. Induction of permeability across endothelial cell monolayers by tumor necrosis factor (TNF) occurs via a tissue factor-dependent mechanism: Relationship between the procoagulant and permeability effects of TNF. Blood 100: 1334–1339.
Petrache, I., A. Birukova, S.I. Ramirez, J.G. Garcia, and A.D. Verin. 2003. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. American Journal of Respiratory Cell and Molecular Biology 28: 574–581.
Andersson, U., H. Wang, K. Palmblad, et al. 2000. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. Journal of Experimental Medicine 192: 565–570.
Hansson, G.K., and P. Libby. 2006. The immune response in atherosclerosis: A double-edged sword. Nature Reviews. Immunology 6: 508–519.
Andersson, U., and K.J. Tracey. 2011. HMGB1 is a therapeutic target for sterile inflammation and infection. Annual Review of Immunology 29: 139–162.
Wang, F.P., L. Li, J. Li, J.Y. Wang, L.Y. Wang, and W. Jiang. 2013. High mobility group box-1 promotes the proliferation and migration of hepatic stellate cells via TLR4-dependent signal pathways of PI3K/Akt and JNK. PLoS One 8, e64373.
Lockyer, J.M., J.S. Colladay, W.L. Alperin-Lea, T. Hammond, and A.J. Buda. 1998. Inhibition of nuclear factor-kappaB-mediated adhesion molecule expression in human endothelial cells. Circulation Research 82: 314–320.
Rose, B.A., T. Force, and Y. Wang. 2010. Mitogen-activated protein kinase signaling in the heart: Angels versus demons in a heart-breaking tale. Physiological Reviews 90: 1507–1546.
Park, J.S., F. Gamboni-Robertson, Q. He, et al. 2006. High mobility group box 1 protein interacts with multiple Toll-like receptors. American Journal of Physiology - Cell Physiology 290: C917–C924.
Yang, H., and K.J. Tracey. 2010. Targeting HMGB1 in inflammation. Biochimica et Biophysica Acta 1799: 149–156.
Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420: 885–891.
Bhatia, M., M. He, H. Zhang, and S. Moochhala. 2009. Sepsis as a model of SIRS. Frontiers in Bioscience 14: 4703–4711.
Tracey, K.J., Y. Fong, D.G. Hesse, et al. 1987. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330: 662–664.
Wichterman, K.A., A.E. Baue, and I.H. Chaudry. 1980. Sepsis and septic shock—a review of laboratory models and a proposal. Journal of Surgical Research 29: 189–201.
Wang, H., H. Yang, C.J. Czura, A.E. Sama, and K.J. Tracey. 2001. HMGB1 as a late mediator of lethal systemic inflammation. American Journal of Respiratory and Critical Care Medicine 164: 1768–1773.
Ramachandran, G. 2014. Gram-positive and gram-negative bacterial toxins in sepsis: A brief review. Virulence 5: 213–218.
Tanida, N., N. Ohno, Y. Adachi, et al. 1992. Modification of immunopharmacological activities of synthetic monosaccharide lipid A analogue, GLA60, by lysozyme. Journal of Biochemistry 112: 616–623.
Takada, K., N. Ohno, and T. Yadomae. 1994. Binding of lysozyme to lipopolysaccharide suppresses tumor necrosis factor production in vivo. Infection and Immunity 62: 1171–1175.
Ohno, N., and D.C. Morrison. 1989. Lipopolysaccharide interaction with lysozyme. Binding of lipopolysaccharide to lysozyme and inhibition of lysozyme enzymatic activity. Journal of Biological Chemistry 264: 4434–4441.
Thachil, J., C.H. Toh, M. Levi, and H.G. Watson. 2012. The withdrawal of Activated Protein C from the use in patients with severe sepsis and DIC [Amendment to the BCSH guideline on disseminated intravascular coagulation]. British Journal of Haematology 157: 493–494.
Acknowledgments
This study was supported by the National Research Foundation of Korea (NRF) funded by the Korean government [MSIP] (Grant No. 2012R1A5A2A42671316) and by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI14C2202).
Conflict of Interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wonhwa Lee and Sae-Kwang Ku contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lee, W., Ku, SK., Na, D.H. et al. Anti-Inflammatory Effects of Lysozyme Against HMGB1 in Human Endothelial Cells and in Mice. Inflammation 38, 1911–1924 (2015). https://doi.org/10.1007/s10753-015-0171-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0171-8