Abstract
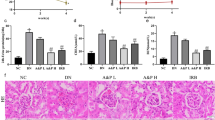
As one of the most important long-term complications of diabetes, diabetic nephropathy (DN) is the major cause of end-stage renal disease and high mortality in diabetic patients. The long pentraxin 3 (Ptx3) is a member of a superfamily of conserved proteins characterized by a cyclic multimeric structure and a conserved C-terminal domain. Several clinical investigations have demonstrated that elevated plasma Ptx3 levels are associated with cardiovascular and chronic kidney diseases (CKD). However, the therapeutic effect of Ptx3 on DN has never been investigated. In our current study, we showed a crucial role for Ptx3 in attenuating renal damage in DN. In our mouse hyperglycemia-induced nephropathy model, Ptx3 treatment showed significantly increased expression of nephrin, acetylated nephrin, and Wilm’s tumor-1 protein (WT-1) when compared with control. The number of CD4+ T cells, CD8+ T cells, Ly6G+ neutrophils, and CD11b+ macrophages were all significantly lower in the Ptx3-treated group than that in the control group in DN. The IL-4 and IL-13 levels in the Ptx3-treated group were markedly higher than that in the control group in DN. Correspondingly, the Ptx3-treated group showed increased numbers of Arg1- or CD206-expressing macrophages compared with the control group. Furthermore, inhibition of Ptx3-treated macrophages abrogated the alleviated renal damage induced by Ptx3 treatment. In conclusion, Ptx3 attenuates renal damage in DN by promoting M2 macrophage differentiation.






Similar content being viewed by others
References
Dronavalli, S., I. Duka, and G.L. Bakris. 2008. The pathogenesis of diabetic nephropathy. Nature Clinical Practice Endocrinology & Metabolism 4: 444–452.
Alvarez, M.L., and J.K. Distefano. 2013. The role of non-coding RNAs in diabetic nephropathy: potential applications as biomarkers for disease development and progression. Diabetes Research and Clinical Practice 99: 1–11.
Sun, Y.M., Y. Su, J. Li, and L.F. Wang. 2013. Recent advances in understanding the biochemical and molecular mechanism of diabetic nephropathy. Biochemical and Biophysical Research Communications 433: 359–361.
Bozza, S., F. Bistoni, R. Gaziano, L. Pitzurra, T. Zelante, P. Bonifazi, et al. 2006. Pentraxin 3 protects from MCMV infection and reactivation through TLR sensing pathways leading to IRF3 activation. Blood 108: 3387–3396.
Garlanda, C., B. Bottazzi, A. Bastone, and A. Mantovani. 2005. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annual Review of Immunology 23: 337–366.
Cieslik, P., and A. Hrycek. 2012. Long pentraxin 3 (PTX3) in the light of its structure, mechanism of action and clinical implications. Autoimmunity 45: 119–128.
Yamasaki, K., M. Kurimura, T. Kasai, M. Sagara, T. Kodama, and K. Inoue. 2009. Determination of physiological plasma pentraxin 3 (PTX3) levels in healthy populations. Clinical Chemistry and Laboratory Medicine 47: 471–477.
Dubin, R., Y. Li, J.H. Ix, M.G. Shlipak, M. Whooley, and C.A. Peralta. 2012. Associations of pentraxin-3 with cardiovascular events, incident heart failure, and mortality among persons with coronary heart disease: data from the Heart and Soul Study. American Heart Journal 163: 274–279.
Tong, M., J.J. Carrero, A.R. Qureshi, B. Anderstam, O. Heimburger, P. Barany, et al. 2007. Plasma pentraxin 3 in patients with chronic kidney disease: associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clinical Journal of the American Society of Nephrology 2: 889–897.
Suliman, M.E., M.I. Yilmaz, J.J. Carrero, A.R. Qureshi, M. Saglam, O.M. Ipcioglu, et al. 2008. Novel links between the long pentraxin 3, endothelial dysfunction, and albuminuria in early and advanced chronic kidney disease. Clinical Journal of the American Society of Nephrology 3: 976–985.
Meuwese, C.L., J.J. Carrero, and P. Stenvinkel. 2011. Recent insights in inflammation-associated wasting in patients with chronic kidney disease. Contributions to Nephrology 171: 120–126.
Daigo, K., M. Nakakido, R. Ohashi, R. Fukuda, K. Matsubara, T. Minami, et al. 2014. Protective effect of the long pentraxin PTX3 against histone-mediated endothelial cell cytotoxicity in sepsis. Science Signaling 7: a88.
Miyamoto, T., Q.A. Rashid, O. Heimburger, P. Barany, K. Carrero, B. Sjoberg, et al. 2011. Inverse relationship between the inflammatory marker pentraxin-3, fat body mass, and abdominal obesity in end-stage renal disease. Clinical Journal of the American Society of Nephrology 6: 2785–2791.
Osorio-Conles, O., M. Guitart, M.R. Chacon, E. Maymo-Masip, J.M. Moreno-Navarrete, M. Montori-Grau, et al. 2011. Plasma PTX3 protein levels inversely correlate with insulin secretion and obesity, whereas visceral adipose tissue PTX3 gene expression is increased in obesity. American Journal of Physiology Endocrinology and Metabolism 301: E1254–E1261.
Abu, S.N., A. Witasp, M.W. Wan, B. Anderstam, K. Brismar, P. Stenvinkel, et al. 2013. Evaluation of the association of plasma pentraxin 3 levels with type 2 diabetes and diabetic nephropathy in a Malay population. Journal of Diabetes Research 2013: 298019.
Pichaiwong, W., K.L. Hudkins, T. Wietecha, T.Q. Nguyen, C. Tachaudomdach, W. Li, et al. 2013. Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. Journal of the American Society of Nephrology 24: 1088–1102.
Yuen, D.A., B.E. Stead, Y. Zhang, K.E. White, M.G. Kabir, K. Thai, et al. 2012. eNOS deficiency predisposes podocytes to injury in diabetes. Journal of the American Society of Nephrology 23: 1810–1823.
Inoki, K., H. Mori, J. Wang, T. Suzuki, S. Hong, S. Yoshida, et al. 2011. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. The Journal of Clinical Investigation 121: 2181–2196.
George, B., R. Verma, A.A. Soofi, P. Garg, J. Zhang, T.J. Park, et al. 2012. Crk1/2-dependent signaling is necessary for podocyte foot process spreading in mouse models of glomerular disease. The Journal of Clinical Investigation 122: 674–692.
Kajiho, Y., Y. Harita, H. Kurihara, S. Horita, A. Matsunaga, H. Tsurumi, et al. 2012. SIRPalpha interacts with nephrin at the podocyte slit diaphragm. The FEBS Journal 279: 3010–3021.
Lin, C.L., P.H. Lee, Y.C. Hsu, C.C. Lei, J.Y. Ko, P.C. Chuang, et al. 2014. MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction. Journal of the American Society of Nephrology 25: 1698–1709.
Wilson, H.M., D. Walbaum, and A.J. Rees. 2004. Macrophages and the kidney. Current Opinion in Nephrology and Hypertension 13: 285–290.
Eardley, K.S., D. Zehnder, M. Quinkler, J. Lepenies, R.L. Bates, C.O. Savage, et al. 2006. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney International 69: 1189–1197.
Ogawa, D., K. Shikata, M. Matsuda, S. Okada, J. Wada, S. Yamaguchi, et al. 2002. Preventive effect of sulphated colominic acid on P-selectin-dependent infiltration of macrophages in experimentally induced crescentic glomerulonephritis. Clinical and Experimental Immunology 129: 43–53.
Wang, Y., Y.P. Wang, G. Zheng, V.W. Lee, L. Ouyang, D.H. Chang, et al. 2007. Ex vivo programmed macrophages ameliorates experimental chronic inflammatory renal disease. Kidney International 72: 290–299.
Cao, Q., C. Wang, D. Zheng, Y. Wang, V.W. Lee, Y.M. Wang, et al. 2011. IL-25 induces M2 macrophages and reduces renal injury in proteinuric kidney disease. Journal of the American Society of Nephrology 22: 1229–1239.
Ricardo, S.D., H. van Goor, and A.A. Eddy. 2008. Macrophage diversity in renal injury and repair. The Journal of Clinical Investigation 118: 3522–3530.
Martinez, F.O., L. Helming, and S. Gordon. 2009. Alternative activation of macrophages: an immunologic functional perspective. Annual Review of Immunology 27: 451–483.
Wynn, T.A. 2004. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nature Reviews. Immunology 4: 583–594.
Bouhlel, M.A., B. Derudas, E. Rigamonti, R. Dievart, J. Brozek, S. Haulon, et al. 2007. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metabolism 6: 137–143.
Zhang, M.Z., B. Yao, S. Yang, L. Jiang, S. Wang, X. Fan, et al. 2012. CSF-1 signaling mediates recovery from acute kidney injury. The Journal of Clinical Investigation 122: 4519–4532.
Takeda, Y., S. Costa, E. Delamarre, C. Roncal, D.O.R. Leite, M.L. Squadrito, et al. 2011. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature 479: 122–126.
Lee, S., S. Huen, H. Nishio, S. Nishio, H.K. Lee, B.S. Choi, et al. 2011. Distinct macrophage phenotypes contribute to kidney injury and repair. Journal of the American Society of Nephrology 22: 317–326.
Conflicts of interests
The authors declare that there is no conflict of interests regarding the publication of this paper
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, H., Tian, J., Xian, W. et al. Pentraxin-3 Attenuates Renal Damage in Diabetic Nephropathy by Promoting M2 Macrophage Differentiation. Inflammation 38, 1739–1747 (2015). https://doi.org/10.1007/s10753-015-0151-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0151-z