Abstract
Embelin has been used to treat fever and inflammatory diseases for thousands of years. Although reports indicate that embelin has antiinflammatory effects, its effects on myocardial injury following cardiac arrest (CA) have not been previously explored. In this study, we aim to investigate the protective effects of embelin on myocardial ischemia–reperfusion injury (IRI) following CA in a rabbit model. Pro-inflammatory (TNF-α, IL-1β, and IL-6) cytokines, cardiac troponin I (cTnI), necrosis ratio, apoptotic index (AI), hemodynamics, nuclear factor-kappa B (NF-κB) p65, and histological damage have been measured or evaluated. Embelin reverts TNF-α, IL-1β, and IL-6 to basal levels and reduces the serum level of cTnI, the necrosis ratio, the AI, and the expression of NF-κB p65. Meanwhile, it improves the hemodynamics and myocardial function. Moreover, embelin-treated groups also showed improved myocardial morphology. Our results indicate that embelin may protect the heart against myocardial IRI following CA via its antiinflammatory abilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Significant progress in resuscitation has improved the restoration of spontaneous circulation (ROSC) in cardiac arrest (CA) victims. Disappointingly, however, the overall survival to discharge remains dismally low at 9.6–17 % for out-of-hospital cardiac arrest (OHCA) and in-hospital cardiac arrest (IHCA) [1]. It has been demonstrated that the systemic ischemia/reperfusion injury (IRI) occurring after CA is a major factor that causes the poor outcome [2]. Endothelial activation and neutrophil extravasation following IRI induces inflammatory cascade activation, finally triggers the systematic inflammatory response syndrome (SIRS), and may lead to myocardial dysfunction and multiple organ failure (MOF) [3]. It is reasonable to surmise that suppressing inflammation is essential to improve the prognosis of patients suffering from post-resuscitation disease. Previous studies also support that antiinflammatory measurement is beneficial for myocardial IRI after resuscitation [4].
Embelin is a naturally occurring alkyl substituted hydroxy benzoquinone and a major constituent from all the parts of Embelia ribes Burm. The plant is used traditionally as antiinflammatory to relieve rheumatism and fever [5]. Embelin also reportedly possesses antioxidant [6], hepatoprotective [7], antibacterial [8], antidiabetic [9], and antiinflammatory properties in other organs [10–12]. Moreover, it has been demonstrated that embelin blocks nuclear factor-κB (NF-κB) signaling pathway [13] which is a key control protein in IRI-induced inflammatory reaction. However, to the best of our knowledge, embelin has not been tested so far for its antiinflammatory properties on myocardial IRI following CA. In our study, we investigated whether embelin has a protective effect on myocardial IRI following resuscitation in a rabbit model.
MATERIALS AND METHODS
Animal Preparation
Fifty adult male specific pathogen-free (SPF) New Zealand white rabbits weighing 2.2 to 3.0 kg were purchased from the Animal Bio-safety Level III laboratory (ABSL-III lab) of Wuhan University (Wuhan, China). Experiments were performed in the Center for Animal Experiment of Wuhan University. All rabbits received standardized care in accordance with the National Institute of Health Guidelines for Ethical Animal Research. The research committee of Wuhan University approved this study (ID: 20130057).
Animals were kept in animal experimental room and maintained at 25 ± 5 °C under 12-h light/dark cycles. After an overnight fast with free access to water, the rabbits were anesthetized via the right marginal vein of the ear (30 mg/kg pentobarbital, i.v.). Additional dose of pentobarbital (10 mg/kg body weight) was given hourly to rabbits for maintaining anesthesia. Anesthetized rabbits were fixed at spine position on a warming blanket to ensure the rectum temperature at 38.5 ± 0.5 °C. An inverse T-incision was made at the level of three to four cartilage rings below the thyroid cartilage, and then, a “Y” type endotracheal tube connected to a ventilator (Evita 2 dura, Draeger Medical Equipment Co., Ltd., Shanghai, China) was inserted that is mechanically ventilated with room air at a respiratory rate of 50/min, 10 ml/kg tidal, and compression-to-ventilation ratio of 1:2. Right femoral arterial pressure and left ventricular pressure were monitored with a 16G cardiac catheter connected to BL-420 F biosignal acquisition system (Taimeng, Chengdu, China). The electrocardiograph was continuously recorded.
Study Design
The CPR model was modified from the methods reported by Chen et al. [14]. Fifty rabbits achieved ultimate success and were randomly divided into three main groups: (1) sham group (n = 10), (2) CPR group (n = 10), and (3) embelin group (n = 10 in each subgroup, 3 subgroups in total.). When the baseline hemodynamic was stable, the ventilator in embelin and CPR groups was turned off and the trachea was clamped at the expiratory phase for approximately 5–9 min to induce cardiac arrest (absence of spontaneous pulse, sinus arrest in electrocardiogram (ECG), pulseless electrical activity or ventricular fibrillation, and mean arterial pressure (MAP) ≤10 mmHg). Three minutes after CA, chest compression and mechanical ventilation were initiated. Manual chest compression at a rate of 180 bpm with equal compression–relaxation duration was performed by one investigator. Compression depth was one third of the anteroposterior diameter of the chest. Meanwhile, mechanical ventilation with pure oxygen was performed at 50/min until spontaneous respiration recovered. Chest compression was paused after 2 min to allow for a 5-s evaluation. If resuscitation was not achieved after 2 min of CPR, advanced cardiac life support was started, epinephrine (2 ml, 200 μg/kg in normal saline) was given every 3 min via the marginal vein of the ear, three times maximum. Chest compression was intermittent at 2-min intervals to check for ROSC achievement. If ventricular fibrillation was present during CPR, biphasic defibrillation was performed (10 J) every 2 min until normal sinus rhythm was recovered. ROSC was defined as the systolic arterial pressure ≥60 mmHg for a minimum of 5 min. If ROSC was not achieved 10 min after resuscitation commenced, it was defined as failure of resuscitation.
After ROSC, embelin (1, 10, and 100 mg/kg body weight in subgroups 1–3, respectively) and equivalent volume of saline were given to rabbits in the CPR group, via the marginal vein of the ear within 30 s. For successfully resuscitated rabbits, hemodynamics was monitored for 6 h; blood samples and myocardial tissue were collected for biochemical and histological evaluation.
MEASUREMENTS
Hemodynamic and Electrocardiogram
The heart rate (HR), mean arterial pressure (MAP), left ventricular systolic pressure (LVSP), max rate of left ventricular pressure rise (dP/dt), negative dP/dt, and ECG were continuously displayed and recorded with a biosignal acquisition system (BL-420 F, Taimeng, Chengdu, China) throughout this experiment.
Blood Analysis
Blood samples were collected from the femoral artery for the following measurements: routine blood was measured with a ABX micros CPR 200 (HORIBA, Princeton, USA); at the same time, a fraction of blood was frozen in liquid nitrogen and stored at −80 °C for cardiac troponin I (cTnI) measurement.
Inflammatory Infiltration and Apoptosis
Left ventricular tissue (4 × 4 × 4 mm, close to the apex cordis) was fixed in 4 % paraformaldehyde (1:10), embedded in paraffin, and cut into sections 4 μm in thickness for observation of inflammatory and apoptosis (TUNEL) and calculating the apoptotic index (AI) (each slice count positive apoptotic nuclei of five high-power field/total nuclei, the mean value for the apoptosis index).
Inflammatory Cytokines, cTnI, and NF-κB
Left ventricle tissue (300–500 mg) was cut into pieces on ice and stored in liquid nitrogen. The tissue content of TNF-α, IL-1β and IL-6, and cTnI were determined by rabbit-specific ELISA (OD 450 nm) using commercial kits (Jiamei Biotech, Beijing, China) in homogenates of frozen tissues, and NF-κB p65 expression was determined using Western blot assay in duplicates as described [14]. Anti-NF-kappa B p65 antibody was purchased from Abcam (MA, USA). Protein bands were normalized with GAPDH, and all blots were quantified with Software Quantity One (Bio-Rad).
Myocardial Necrosis Ratio
Left ventricles were sliced transversely from the base to apex into five sections (1–2 mm in thickness), then incubated in 1 % triphenyl tetrazolium chloride (TTC) followed by 4 % paraformaldehyde. The fluid was removed, and the image of the ventricle was captured with a digital camera (Canon IXUS 120 IS, Japan). The total left ventricle area and necrotic area were measured, and the necrosis ratio (necrotic area/total left ventricle area, ×100 %) was calculated.
Statistical Analysis
Data were expressed as mean ± standard deviation. One-way analysis of variance (ANOVA) was used to compare the means among different groups. The SNK test was used to compare the means between two groups if homogeneity of variance was present; Tamhane’s T2 test was used if heterogeneity of variance was present. Repeated-measures ANOVA was used to compare the changes in parameters over time. SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was employed for statistical analysis, and values of P < 0.05 were considered significant.
RESULTS
Hemodynamics in Different Groups
As shown in Fig. 1, MAP, LVSP, and dP/dt were decreased and − dP/dt was increased in all groups at multiple time points compared to prior to resuscitation. At all time points after resuscitation, MAP in the embelin group was higher than that in the CPR group (P < 0.05, respectively). Compared with the CPR group, LVSP in embelin groups was significantly increased at 2, 4, and 6 h after resuscitation (P < 0.05, respectively). In addition, at 0.5–6 h after resuscitation, dP/dt in embelin groups was dramatically higher than that in the CPR group (P < 0.05, respectively), and − dP/dt at 1, 2, 4, and 6 h after resuscitation was significantly lower than that in the CPR group (P < 0.05, respectively). Resuscitation made no changes of HR neither in the CPR group and embelin groups nor between CPR group and embelin groups.
Blood Analysis
As seen in Table 1, the count of neutrophils in the embelin groups and the CPR group was significantly higher than that in the sham group (P < 0.05, respectively). Compared with the CPR group, neutrophil levels in the embelin groups (10 and 100 mg/kg) were significantly decreased (P < 0.05, respectively). However, there were no significant differences among all groups in erythrocytes, hemoglobin, lymphocytes, and platelet levels. The pH and base excess (BE) level reduced in embelin groups and CPR group compared to the sham group (P < 0.05, respectively). The level of serum cTnI was significantly increased in embelin groups and CPR group compared with the sham group and lower in embelin groups compared with CPR group (P < 0.05, respectively).
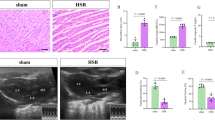
Myocardial Histomorphology, Necrosis, and Apoptosis
According to Fig. 2, we found that myocardial cells in the sham group lined in order and sarcolemma kept integrity, without neutrophil infiltration. In the CPR group, myocardial cells structure was disordered with neutrophils infiltrating, significant apoptosis, and necrosis. Embelin groups showed improved myocardial morphology and decreased necrosis and apoptosis according to the necrosis ratio and apoptotic index (P < 0.05, respectively). In addition, embelin reduced both necrosis and apoptosis in a dose-dependent manner.
Inflammatory Cytokines and NF-κB
The expressions of TNF-α, IL-1β, and IL-6 were significantly lower in embelin groups than those in the CPR group, but higher than those in the sham group (P < 0.05, respectively, Fig. 3). Moreover, embelin inhibited the expression of TNF-α, IL-1β, and IL-6 in a dose-dependent manner. It was clearly shown in Fig. 4 that the expression of NF-κB p65 in the CPR group was significantly higher than that in the sham group and embelin groups (P < 0.05, respectively). In addition, the decreased expression of NF-κB p65 was associated with increased embelin dosages.
DISCUSSION
The aim of the present study is to determine the antiinflammatory reactivity of embelin, a major constituent from Embelia ribes Burm, on myocardial I/R injury following resuscitation. We found that embelin reduced neutrophils and necrosis ratio, downregulated expression of inflammatory cytokines, and showed effect on inhibition of NF-κB and reduction of myocardial apoptosis after cardiac arrest and followed resuscitation in a dose-dependent manner.
Following CA, IRI is one of the major factors that cause considerable mortality and morbidity after ROSC. During reperfusion, excessive activation of neutrophils and vascular endothelial injury increase vascular permeability and recruitment of inflammatory cells, initiating inflammatory cascade reaction [15, 16]. Increasing evidences demonstrate that post-resuscitation abnormalities after cardiac arrest mimic the immunologic and coagulation disorders observed in severe sepsis [17, 18], such as high levels of circulating cytokines and adhesion molecules, presence of plasma endotoxin, and dysregulated leukocyte production of cytokines. A systemic inflammatory response syndrome following IRI after resuscitation is predictive of subsequent multiple organ failure and death [19]. Furthermore, inflammation is one of the most important factors which results in ventricular dysfunction following myocardial IRI [18, 20], and the expression of inflammatory cytokines is highly correlated with the deterioration of myocardial function [21]. Antiinflammatory management should be considered as a potential treatment for patients resuscitated after cardiac arrest. In earlier studies, embelin showed its ability of inhibiting the activation of some pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 in skin inflammation, rats of colitis, or in human glioma cells [11, 22, 23]. Our results are consistent with previous findings in other organs, and we are the first to demonstrate the antiinflammatory effect of embelin in heart.
We also found that embelin possibly mediated its antiinflammatory property by modulating NF-κB which serves as an upstream signaling molecule to regulate the expression of apoptosis-related genes [24]. As the earliest initiation factor of inflammation, NF-κB played a key role in myocardial IRI process [25]. Activated NF-κB induces upregulation of TNF-α leading to IRI such as the expression of inflammatory mediators, recruitment of neutrophils, decrease of myocardial contractility, and induction of myocardial cell apoptosis [26, 27], while suppression of NF-κB reduced myocardial no-reflow phenomenon and improved myocardial function after IRI [28]. In the recent years, studies showed that NF-κB regulates the expression of several apoptosis-related genes, and the activation of NF-κB can prevent cell apoptosis in tumor [29]. Embelin was identified as a cell-permeable, small molecular weight inhibitor of the X chromosome-linked inhibitor-of-apoptosis protein (XIAP), an important function of which is its role in signaling to NF-κB activation [30]. Embelin was reported to potently inhibit NF-κB activation in a dose- and time-dependent manner [13]. Similarly, in our study, the expression of NF-κB in embelin groups was significantly lower than that in the CPR group. Embelin treatment significantly reduced the expression of NF-κB after myocardial IRI, and cell apoptosis was significantly increased in the embelin groups. All those results indicated that embelin has a protective effect on myocardial cells suffering from IRI. Taken together, these results support the notion that embelin possesses antiinflammatory properties and may be a good candidate for the treatment of IRI after CPR.
CONCLUSION
In the present study, for the first time, we reported that embelin has antiinflammatory activities against IRI following resuscitation. Embelin downregulates the expression of pro-inflammatory cytokines. The antiinflammation effect may be due to inhibition of NF-κB. These effects help to decrease apoptosis and necrosis of myocardium, ultimately ameliorate cardiac function following resuscitation, and improve the prognosis.
References
Geocadin, R.G., M.A. Peberdy, and R.M. Lazar. 2012. Poor survival after cardiac arrest resuscitation: a self-fulfilling prophecy or biologic destiny? Crit Care Med 40: 979–80.
Chalkias, A., and T. Xanthos. 2012. Pathophysiology and pathogenesis of post-resuscitation myocardial stunning. Heart Fail Rev 17: 117–28.
Adrie, C., M. Adib-Conquy, I. Laurent, et al. 2002. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation 106: 562–8.
Chen, Z., Z. Wu, C. Huang, et al. 2013. Effect of lipoxin A4 on myocardial ischemia reperfusion injury following cardiac arrest in a rabbit model. Inflammation 36: 468–75.
Kapoor, V.K., A.S. Chawla, M. Kumar, et al. 1983. Anti-inflammatory agent in Indian Laboratories. Indian Drugs 30: 481–488.
Joshi, R., J.P. Kamat, and T. Mukherjee. 2007. Free radical scavenging reactions and antioxidant activity of embelin: biochemical and pulse radiolytic studies. Chem Biol Interact 167: 125–34.
Singh, D., R. Singh, P. Singh, et al. 2009. Effects of embelin on lipid peroxidation and free radical scavenging activity against liver damage in rats. Basic Clin Pharmacol Toxicol 105: 243–8.
Chitra, M., S. Devi, and E. Sukumar. 2003. Antibacterial activity of embelin. Fitoterapia 74: 401–403.
Bhandari, U., and M.N. Ansari. 2009. Ameliorative effect of an ethanol extract of Embelia ribes fruits on isoproterenol-induced cardiotoxicity in diabetic rats. Pharm Biol 47: 669–674.
Chitra, M., E. Sukumar, V. Suja, et al. 1994. Antitumor, anti-inflammatory and analgesic property of embelin, a plant product. Chemotherapy 40: 109–113.
Thippeswamy, B.S1., S. Mahendran, M.I. Biradar, et al. 2011. Protective effect of embelin against acetic acid induced ulcerative colitis in rats. Eur J Pharmacol 654: 100–5.
Kumar, G.K1., R. Dhamotharan, N.M. Kulkarni, et al. 2011. Embelin ameliorates dextran sodium sulfate-induced colitis in mice. Int Immunopharmacol 11: 724–31.
Ahn, K.S., G. Sethi, and B.B. Aggarwal. 2007. Embelin, an inhibitor of X chromosome-linked inhibitor-of-apoptosis protein, blocks nuclear factor-kappaB (NF-kappaB) signaling pathway leading to suppression of NF-kappaB-regulated antiapoptotic and metastatic gene products. Mol Pharmacol 71: 209–19.
Chen, M.H., L. Xie, T.W. Liu, et al. 2007. Epinephrine, but not vasopressin, improves survival rates in an adult rabbit model of asphyxia cardiac arrest. Am J Emerg Med 25: 509–14.
Gross, G.J., and J.A. Auchampach. 2007. Reperfusion injury: does it exist? J Mol Cell Cardiol 42: 12–8.
Jordan, J.E., Z. Zhao, and J. Vinten-Johansen. 1999. The role of neutrophils in myocardial ischemia–reperfusion injury. Cardiovasc Res 43: 860–78.
Adrie, C., I. Laurent, M. Monchi, et al. 2004. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care 10: 208–12.
Laurent, I., M. Monchi, J.D. Chiche, et al. 2002. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol 40: 2110–6.
Nolan, J.P., R.W. Neumar, C. Adrie, et al. 2010. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke (Part II). Int Emerg Nurs 18: 8–28.
Ruiz-Bailén, M., E. Aguayo de Hoyos, S. Ruiz-Navarro, et al. 2005. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation 66: 175–81.
Vinten-Johansen, J1., R. Jiang, J.G. Reeves, et al. 2007. Inflammation, proinflammatory mediators and myocardial ischemia-reperfusion Injury. Hematol Oncol Clin North Am 21: 123–45.
Kalyan Kumar, G., R. Dhamotharan, N.M. Kulkarni, et al. 2011. Embelin reduces cutaneous TNF-alpha level and ameliorates skin edema in acute and chronic model of skin inflammation in mice. Eur J Pharmacol 662: 63–9.
Park, S.Y., S.L. Lim, H.J. Jang, et al. 2013. Embelin induces apoptosis in human glioma cells through inactivating NF-kappaB. J Pharmacol Sci 121: 192–9.
Aggarwal, B.B. 2004. Nuclear factor-kappaB: the enemy within. Cancer Cell 6: 203–8.
Moss, N.C., W.E. Stansfield, M.S. Willis, et al. 2007. IKKbeta inhibition attenuates myocardial injury and dysfunction following acute ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 293: H2248–53.
Gordon, J.W., J.A. Shaw, and L.A. Kirshenbaum. 2011. Multiple facets of NF-kappaB in the heart: to be or not to NF-kappaB. Circ Res 108: 1122–32.
Yang, J., H. Jiang, J. Yang, et al. 2009. Valsartan preconditioning protects against myocardial ischemia-reperfusion injury through TLR4/NF-kappaB signaling pathway. Mol Cell Biochem 330: 39–46.
Zeng, M., H. Yan, Y. Chen, et al. 2012. Suppression of NF-kappaB reduces myocardial no-reflow. PLoS One 7: e47306.
Ni, H., W. Zhao, X. Kong, et al. 2014. NF-kappa B modulation is involved in celastrol induced human multiple myeloma cell apoptosis. PLoS One 9: e95846.
Lewis, J1., E. Burstein, S.B. Reffey, et al. 2004. Uncoupling of the signaling and caspase-inhibitory properties of X-linked inhibitor of apoptosis. J Biol Chem 279: 9023–9.
Acknowledgments
We would like to thank the Center for Animal Experiment for the assistance in animal study.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, ZG., Tang, ZZ., Zhang, WK. et al. Protective Effects of Embelin on Myocardial Ischemia–Reperfusion Injury Following Cardiac Arrest in a Rabbit Model. Inflammation 38, 527–533 (2015). https://doi.org/10.1007/s10753-014-9959-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-9959-1