Abstract
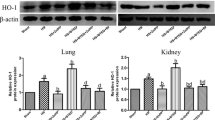
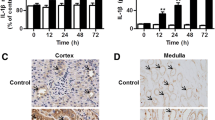
The study was aimed to investigate the potential mechanism of inflammatory renal damage induced by shock wave. A total of 48 rats, with the right kidney cut, are randomly assigned into control group, ESWL group and ESWL + PDTC group. Rats were treated with shock wave at the left kidney. At post-shock wave 3 and 105 days, all the animals were sacrificed for detecting the expression of tumor necrosis factor (TNF)-α, intercellular adhesion molecule (ICAM)-1, and monocyte chemoattractant protein (MCP)-1. The inflammatory responses were evaluated by detecting the level of myeloperoxidase (MPO) and ED-1. The histological renal injury was also examined. Before the animals were sacrificed, the urine samples were collected for measuring the values of malondialdehyde (MDA), β2-microglobulin, interleukin (IL)-6, and IL-18. At post-shock wave 3 days, the higher expression of ICAM-1 and TNF-α were observed in shock wave-treated kidneys. The level of urine TNF-α, IL-6, and IL-18 were also increased significantly. Using PDTC obviously decreased the expression of ICAM-1 and TNF-α. It also effectively inhibited the degree of oxidative stress and neutrophil infiltration. At post-shock wave 105 days, the expression of MCP-1 and the level of urine β2-microglobulin and IL-18 were increased significantly. The histological analysis also indicated more ED-1-positive cells and serious fibrosis in shock wave-treated kidneys. PDTC significantly suppressed MCP-1 and IL-18 expression, decreased monocyte infiltration, and alleviate the degree of interstitium fibrosis. Shock wave triggered excessive inflammatory responses and aggravated renal biological damage. Several inflammatory factors including ICAM-1, MCP-1, and TNF-α were considered to play important role in this type of renal damage.




Similar content being viewed by others
References
Handa, R.K., and A.P. Evan. 2010. A chronic outcome of shock wave lithotripsy is parenchymal fibrosis. Urological Research 38: 301–305.
Goktas, C., A. Coskun, Z. Bicik, R. Horuz, I. Unsal, M. Serteser, S. Albayrak, and K. Sarıca. 2012. Evaluating ESWL-induced renal injury based on urinary TNF-α, IL-1α, and IL-6 levels. Urological Research 40: 569–573.
Howard, D., and B. Sturtevant. 1997. In vitro study of the mechanical effects of shock-wave lithotripsy. Ultrasound in Medicine & Biology 23: 1107–1122.
Li, X., Y. Xue, D. He, X. Chen, and L. Zhang. 2010. Shock wave induces chronic renal lesion through activation of the nuclear factor kappa B signaling pathway. World Journal of Urology 28: 657–662.
Yoshida, T., H. Kumagai, T. Kohsaka, and N. Ikegaya. 2013. Relaxin protects against renal ischemia–reperfusion injury. American Journal of Physiology. Renal Physiology 305: F1169–1176.
Kim, H.J., J.S. Lee, A. Kim, S. Koo, H.J. Cha, J.A. Han, Y. Do, K.M. Kim, B.S. Kwon, R.S. Mittler, H.R. Cho, and B. Kwon. 2013. TLR2 signaling in tubular epithelial cells regulates NK cell recruitment in kidney ischemia–reperfusion injury. Journal of Immunology 191: 2657–2664.
Qiao, X., R.S. Li, H. Li, G.Z. Zhu, X.G. Huang, S. Shao, and B. Bai. 2013. Intermedin protects against renal ischemia–reperfusion injury by inhibition of oxidative stress. American Journal of Physiology. Renal Physiology 304: F112–119.
Kim, J., and B.J. Padanilam. 2011. Loss of poly (ADP-ribose) polymerase 1 attenuates renal fibrosis and inflammation during unilateral ureteral obstruction. American Journal of Physiology. Renal Physiology 301: F450–459.
Ellerbroek, P.M., R.G. Schoemaker, R. van Veghel, A.I. Hoepelman, and F.E. Coenjaerts. 2004. Cryptococcal capsular glucuronoxylomannan reduces ischaemia-related neutrophil influx. European Journal of Clinical Investigation 34: 631–640.
Ghosh, S.S., R. Krieg, H.D. Massey, D.A. Sica, I. Fakhry, S. Ghosh, and W. Gehr. 2012. Curcumin and enalapril ameliorate renal failure by antagonizing inflammation in 5⁄6 nephrectomized rats: role of phospholipase and cyclooxygenase. American Journal of Physiology. Renal Physiology 302: F439–454.
Clark, D.L., B.A. Connors, R.K. Handa, and A.P. Evan. 2011. Pretreatment with low-energy shock waves reduces the renal oxidative stress and inflammation caused by high-energy shock wave lithotripsy. Urological Research 39: 437–42.
Clark, D.L., B.A. Connors, and A.P. Evan. 2011. Effect of shock wave number on renal oxidative stress and inflammation. BJU International 107: 318–322.
Ng, C.F., A.K. Lo, K.W. Lee, K.T. Wong, W.Y. Chung, and D. Gohel. 2012. A prospective, randomized study of the clinical effects of shock wave delivery for unilateral kidney stones: 60 versus 120 shocks per minute. The Journal of Urology 188: 837–842.
Chargui, I., I. Grissa, F. Bensassi, M.Y. Hrira, S. Haouem, Z. Haouas, and H. Bencheikh. 2012. Oxidative stress, biochemical and histopathological alterations in the liver and kidney of female rats exposed to low doses of deltamethrin (DM): a molecular assessment. Biomedical and Environmental Sciences 25: 672–683.
Huang, C.F., S.H. Liu, and S.Y. Lin-Shiau. 2012. Pyrrolidine dithiocarbamate augments Hg (2+)-mediated induction of macrophage cell death via oxidative stress-induced apoptosis and necrosis signaling pathways. Toxicology Letters 214: 33–45.
Li, Y., J. Wang, X. Zhu, Q. Feng, X. Li, and X. Feng. 2012. Urinary protein markers predict the severity of renal histological lesions in children with mesangial proliferative glomerulo-nephritis. BMC Nephrology 13: 29.
Zubowska, M., K. Wyka, W. Fendler, W. Młynarski, and B. Zalewska-Szewczyk. 2013. Interleukin 18 as a marker of chronic nephropathy in children after anticancer treatment. Disease Markers 35: 811–818.
Cynis, H., A. Kehlen, M. Haegele, T. Hoffmann, U. Heiser, M. Fujii, Y. Shibazaki, H. Yoneyama, S. Schilling, and H.U. Demuth. 2013. Inhibition of glutaminyl cyclases alleviates CCL2-mediated inflammation of non-alcoholic fatty liver disease in mice. International Journal of Experimental Pathology 94: 217–225.
Liu, Z.H., L.L. Chen, X.L. Deng, H.J. Song, Y.F. Liao, T.S. Zeng, J. Zheng, and H.Q. Li. 2012. Methylation status of CpG sites in the MCP-1 promoter is correlated to serum MCP-1 in type 2 diabetes. Journal of Endocrinological Investigation 35: 585–589.
Han, Y., F.Y. Ma, G.H. Tesch, C.L. Manthey, and D.J. Nikolic Paterson. 2013. Role of macrophages in the fibrotic phase of rat crescentic glomerulonephritis. American Journal of Physiology. Renal Physiology 304: F1043–1053.
Farris, A.B., and R.B. Colvin. 2012. Renal interstitial fibrosis: mechanisms and evaluation. Current Opinion in Nephrology and Hypertension 21: 289–300.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Grant No. 30700833).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Long, Q., Cheng, X. et al. Shock Wave Induces Biological Renal Damage by Activating Excessive Inflammatory Responses in Rat Model. Inflammation 37, 1317–1325 (2014). https://doi.org/10.1007/s10753-014-9859-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-9859-4