Abstract
The accumulation of advanced oxidation protein products (AOPPs) has been linked to several pathological conditions, and their levels are formed during oxidative stress as a result of reactions between plasma proteins and chlorinated oxidants produced by myeloperoxidase (MPO). However, it was suggested that the generation of this mediator of inflammation may also occur via an MPO-independent pathway. The aim of this study was to induce the formation of AOPPs in vitro through Fenton reaction and to investigate whether this generation could be counteracted by N-acetylcysteine (NAC) and fructose-1,6-bisphosphate (FBP). The complete Fenton system increased the AOPPs levels and both NAC and FBP were capable of inhibiting the formation of Fenton reaction-induced AOPPs. These data provide a new hypothesis about another pathway of AOPPs formation, as well as report that NAC and FBP may be good candidates to neutralize pro-inflammatory and pro-oxidant effects of AOPPs in several diseases.





Similar content being viewed by others
References
Halliwell, B. 2012. Free radicals and antioxidants: updating a personal view. Nutrition Reviews 70: 257–265.
Ray, P.D., B.W. Huang, and Y. Tsuji. 2012. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular Signalling 24: 981–990.
Sorescu, D., D. Weiss, B. Lassegue, R.E. Clempus, K. Szocs, G.P. Sorescu, L. Valppu, M.T. Quinn, J.D. Lambeth, J.D. Vega, W.R. Taylor, and K.K. Griendling. 2002. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105: 1429–1435.
Brownlee, M. 2001. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820.
Kaneda, H., J. Taguchi, K. Ogasawara, T. Aizawa, and M. Ohno. 2002. Increased level of advanced oxidation protein products in patients with coronary artery disease. Atherosclerosis 162: 221–225.
Kalousova, M., J. Skrha, and T. Zima. 2002. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiological Research 51: 597–604.
Witko-Sarsat, V., M. Friedlander, C. Capeillere-Blandin, T. Nguyen-Khoa, A.T. Nguyen, J. Zingraff, P. Jungers, and B. Descamps-Latscha. 1996. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney International 49: 1304–1313.
Guo, Z.J., H.X. Niu, F.F. Hou, L. Zhang, N. Fu, R. Nagai, X. Lu, B.H. Chen, Y.X. Shan, J.W. Tian, R.H. Nagaraj, D. Xie, and X. Zhang. 2008. Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signaling pathway. Antioxidants and Redox Signaling 10: 1699–1712.
Zhou, L.L., W. Cao, C. Xie, J. Tian, Z. Zhou, Q. Zhou, P. Zhu, A. Li, Y. Liu, T. Miyata, F.F. Hou, and J. Nie. 2012. The receptor of advanced glycation end products plays a central role in advanced oxidation protein products-induced podocyte apoptosis. Kidney International 82: 759–770.
Himmelfarb, J., and E. McMonagle. 2001. Albumin is the major plasma protein target of oxidant stress in uremia. Kidney International 60: 358–363.
Mera, K., M. Anraku, K. Kitamura, K. Nakajou, T. Maruyama, and M. Otagiri. 2005. The structure and function of oxidized albumin in hemodialysis patients: Its role in elevated oxidative stress via neutrophil burst. Biochemical and Biophysical Research Communications 334: 1322–1328.
Shi, X.Y., F.F. Hou, H.X. Niu, G. Wang, D. Xie, Z.J. Guo, Z.M. Zhou, F. Yang, J.W. Tian, and X. Zhang. 2008. Advanced oxidation protein products promote inflammation in diabetic kidney through activation of renal nicotinamide adenine dinucleotide phosphate oxidase. Endocrinology 149: 1829–1839.
Zhou, L.L., F.F. Hou, G.B. Wang, F. Yang, D. Xie, Y.P. Wang, and J.W. Tian. 2009. Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms. Kidney International 76: 1148–1160.
Hanasand, M., R. Omdal, K.B. Norheim, L.G. Gøransson, C. Brede, and G. Jonsson. 2012. Improved detection of advanced oxidation protein products in plasma. Clinica Chimica Acta 413: 901–906.
Capeillère-Blandin, C., V. Gausson, B. Descamps-Latscha, and V. Witko-Sarsat. 2004. Biochemical and spectrophotometric significance of advanced oxidized protein products. Biochimica et Biophysica Acta 1689: 91–102.
Capeillère-Blandin, C., V. Gausson, A.T. Nguyen, B. Descamps-Latscha, T. Drüeke, and V. Witko-Sarsat. 2006. Respective role of uraemic toxins and myeloperoxidase in the uraemic state. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association 21: 1555–1563.
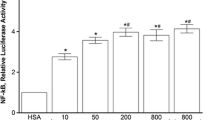
Bochi, G.V., V.D. Torbitz, L.P. Cargnin, M.B. Sangoi, R.C. Santos, P. Gomes, and R.N. Moresco. 2012. Fructose-1,6-bisphosphate and N-acetylcysteine attenuate the formation of advanced oxidation protein products, a new class of inflammatory mediators, in vitro. Inflammation 35: 1786–1792.
Holmes, R.S., and C.J. Masters. 1970. Epigenetic interconversion of the multiple forms of mouse liver catalase. FEBS Letters 11: 45–48.
Witko-Sarsat, V., T. Nguyen Khoa, P. Jungers, T. Drüeke, and B. Descamps-Latscha. 1998. Advanced oxidation protein products: oxidative stress markers and mediators of inflammation in uremia. Advances in nephrology from the Necker Hospital 28: 321–341.
Kalousová, M., J. Skrha, and T. Zima. 2002. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiological Research 51: 597–604.
Martín-Gallán, P., A. Carrascosa, M. Gussinyé, and C. Domínguez. 2003. Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radical Biology and Medicine 34: 1563–1574.
Tabak, O., R. Gelisgen, H. Erman, F. Erdenen, C. Muderrisoglu, H. Aral, and H. Uzun. 2011. Oxidative lipid, protein, and DNA damage as oxidative stress markers in vascular complications of diabetes mellitus. Clinical and Investigative Medicine 34: 163–171.
Chen, S., L. Liu, X. Sun, Y. Liu, and T. Son. 2005. Captopril restores endothelium-dependent relaxation induced by advanced oxidation protein products in rat aorta. Journal of Cardiovascular Pharmacology 46: 803–809.
Drüeke, T., V. Witko-Sarsat, Z. Massy, B. Descamps-Latscha, A.P. Guerin, S.J. Marchais, V. Gausson, and G.M. London. 2002. Iron therapy, advanced oxidation protein products, and carotid artery intima-media thickness in end-stage renal disease. Circulation 106: 2212–2217.
Anraku, M., K. Kitamura, R. Shintomo, K. Takeuchi, H. Ikeda, J. Nagano, T. Ko, K. Mera, K. Tomita, and M. Otagiri. 2008. Effect of intravenous iron administration frequency on AOPP and inflammatory biomarkers in chronic hemodialysis patients: a pilot study. Clinical Biochemistry 41: 1168–1174.
Halliwell, B. 1978. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Its role in degradation of hyaluronic acid by a superoxide-generating system. FEBS Letters 96: 238–242.
Rooyakkers, T.M., E.S. Stroes, M.P. Kooistra, E.E. Van Faassen, R.C. Hider, T.J. Rabelink, and J.J. Marx. 2002. Ferric saccharate induces oxygen radical stress and endothelial dysfunction in vivo. European Journal of Clinical Investigation 32: 9–16.
Tovbin, D., D. Mazor, M. Vorobiov, C. Chaimovitz, and N. Meyerstein. 2002. Induction of protein oxidation by intravenous iron in hemodialysis patients: Role of inflammation. American Journal of Kidney Diseases 40: 1005–1012.
Stępniak, J., A. Lewiński, and M. Karbownik-Lewińska. 2013. Membrane lipids and nuclear DNA are differently susceptive to Fenton reaction substrates in porcine thyroid. Toxicology In Vitro 27: 71–78.
Valko, M., C.J. Rhodes, J. Moncol, M. Izakovic, and M. Mazur. 2006. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions 160: 1–40.
Repka, T., and R.P. Hebbel. 1991. Hydroxyl radical formation by sickle erythrocyte membranes: Role of pathologic iron deposits and cytoplasmic reducing agents. Blood 78: 2753–2758.
Trachootham, D., W. Lu, M.A. Ogasawara, R.D. Nilsa, and P. Huang. 2008. Redox regulation of cell survival. Antioxidants and Redox Signaling 10: 1343–1374.
Pellegrino, P., B. Mallet, S. Delliaux, Y. Jammes, R. Gieu, and O. Schaf. 2011. Zeolites are effective ROS-scavengers in vitro. Biochemical and Biophysical Research Communications 410: 478–483.
Witko-Sarsat, V., V. Gausson, A.T. Nguyen, M. Touam, T. Drüeke, F. Santangelo, and B. Descamps-Latscha. 2003. AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: A potential target for N-acetylcysteine treatment in dialysis patients. Kidney International 64: 82–91.
Aruoma, O.I., B. Halliwell, B.M. Hoey, and J. Butler. 1989. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radical Biology and Medicine 6: 593–597.
Fishbane, S. 2008. N-Acetylcysteine in the prevention of contrast-induced nephropathy. Clinical Journal of the American Society of Nephrology 3: 281–287.
Holt, S., R. Marley, B. Fernando, D. Harry, R. Anand, D. Goodier, and K. Moore. 1999. Acute cholestasis-induced renal failure: Effects of antioxidants and ligands for the thromboxane A2 receptor. Kidney International 55: 271–277.
Santos, R.C., R.N. Moresco, M.A. Peña Rico, A.R. Susperregui, J.L. Rosa, R. Bartrons, F. Ventura, D.N. Mário, S.H. Alves, E. Tatsch, H. Kober, R.O. de Mello, P. Scherer, H.B. Dias, and J.R. de Oliveira. 2012. Fructose-1,6-bisphosphate reduces the mortality in Candida albicans bloodstream infection and prevents the septic-induced platelet decrease. Inflammation 35: 1256–1261.
de Mello, R.O., A. Lunardelli, E. Caberlon, C.M. de Moraes, R. Christ Vianna Santos, V.L. da Costa, G.V. da Silva, P. da Silva Scherer, L.E. Buaes, D.A. da Silva Melo, M.V. Donadio, F.B. Nunes, and J.R. de Oliveira. 2011. Effect of N-acetylcysteine and fructose-1,6-bisphosphate in the treatment of experimental sepsis. Inflammation 34: 539–550.
Alva, N., T. Carbonell, T. Roig, J. Bermúdez, and J. Palomeque. 2011. Fructose 1,6 biphosphate administration to rats prevents metabolic acidosis and oxidative stress induced by deep hypothermia and rewarming. European Journal of Pharmacology 659: 259–264.
Bajić, A., J. Zakrzewska, D. Godjevac, P. Andjus, D.R. Jones, M. Spasić, and I. Spasojević. 2011. Relevance of the ability of fructose 1,6-bis(phosphate) to sequester ferrous but not ferric ions. Carbohydrate Research 346: 416–420.
Wei, X.F., Q.G. Zhou, F.F. Hou, B.Y. Liu, and M. Liang. 2009. Advanced oxidation protein products induce mesangial cell perturbation through PKC-dependent activation of NADPH oxidase. American Journal of Physiology. Renal Physiology 296: 427–437.
Zhou, Q.G., M. Zhou, A.J. Lou, D. Xie, and F.F. Hou. 2010. Advanced oxidation protein products induce inflammatory response and insulin resistance in cultured adipocytes via induction of endoplasmic reticulum stress. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 26: 775–786.
Cao, W., J. Xu, Z.M. Zhou, G.B. Wang, F.F. Hou, and J. Nie. 2013. Advanced oxidation protein products activate intrarenal renin–angiotensin system via a CD36-mediated, redox-dependent pathway. Antioxidants and Redox Signaling 18: 19–35.
Valente, A.J., T. Yoshida, R.A. Clark, P. Delafontaine, U. Siebenlist, and B. Chandrasekar. 2013. Advanced oxidation protein products induce cardiomyocyte death via Nox2/Rac1/superoxide-dependent TRAF3IP2/JNK signaling. Free Radical Biology and Medicine 60: 125–135.
Prousek, J. 2007. Fenton chemistry in biology and medicine. Pure and applied chemistry 79: 2325–2338.
Repetto, M.G., N.F. Ferrarotti, and A. Boveris. 2010. The involvement of transition metal ions on iron-dependent lipid peroxidation. Archives of Toxicology 84: 255–262.
González, F.B., S. Llesuy, and A. Boveris. 1991. Hydroperoxide-initiated chemiluminescence: Assay for oxidative stress in biopsies of heart, liver and muscle. Free Radical Biology and Medicine 10: 93–100.
Chance, B., H. Sies, and A. Boveris. 1979. Hydroperoxide metabolism in mammalian organs. Physiological Reviews 59: 527–605.
Marsche, G., S. Frank, A. Hrzenjak, M. Holzer, S. Dirnberger, C. Wadsack, H. Scharnagl, T. Stojakovic, A. Heinemann, and K. Oettl. 2009. Plasma-advanced oxidation products are potent high-density lipoprotein receptor antagonists in vivo. Circulation Research 104: 750–757.
Acknowledgments
This study was supported by grants to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes, Brazil). We thank SPi Global Professional Editing Services for writing assistance.
Conflict of interest
There are no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bochi, G.V., Torbitz, V.D., Cargnin, L.P. et al. An Alternative Pathway Through the Fenton Reaction for the Formation of Advanced Oxidation Protein Products, a New Class of Inflammatory Mediators. Inflammation 37, 512–521 (2014). https://doi.org/10.1007/s10753-013-9765-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-013-9765-1