Abstract
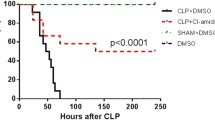
Immune dysfunction is a major cause of mortality in septic patients. Current evidence indicates an important role for dendritic cells (DCs) in the pathophysiology of immune dysfunction, and these cells are potential targets of immunomodulation therapies. In the present study, our aim was to enhance the resistance of endotoxemic mice to bacterial translocation and secondary infection and to improve the outcome of these infections using a combination therapy consisting of thymosin alpha1 and dexamethasone in a timely manner according to the changes of DCs’ number. The effect of treatment with dexamethasone (DXM) and thymosin alpha1 (Tα1) on DCs was investigated by examining their number, MHCII and CD86 expression and their capacity to induce T cell activation. Endotoxemic mice were randomly divided into five treatment groups. The survival rates, the levels of TNF-α and IL-10, the occurrence of bacterial translocation, and the ability to clear secondary infections were determined. Additionally, the behavior of DCs over time was also evaluated. Tα1 induced significant increases in DC numbers in vivo, whereas DXM reduced cell numbers both in vitro and in vivo. However, neither drug induced significant changes in the capacity of DCs to induce T cell activation or their expression of MHCII or CD86. Among the five treatment groups, the mice treated with a combination of DXM and Tα1 had the highest survival rate; this increased survival was associated with a decrease in bacterial translocation to extra-intestinal organs and an enhanced ability to eradicate secondary infections by reversing the change in DC numbers during endotoxemia. Immunomodulatory therapy that combines Tα1 and DXM in a timely manner and was based on changes in DCs enhanced the resistance of endotoxemic mice to bacterial translocation and secondary infections, improving the outcome of the infection.








Similar content being viewed by others
References
Angus, D.C., W.T. Linde-Zwirble, J. Lidicked, G. Clermont, J. Carcillo, and M.R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine 29: 1303–1310.
Martin, G.S., D.M. Mannino, S. Eaton, and M. Moss. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. New England Journal of Medicine 348: 1546–1554.
Levy, M.M., R.P. Dellinger, S.R. Townsend, W.T. Linde-Zwirble, J.C. Marshall, J. Bion, C. Schorr, A. Artigas, G. Ramsay, R. Beale, M.M. Parker, H. Gerlach, K. Reinhart, E. Silva, M. Harvey, S. Regan, and D.C. Angus. 2010. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Medicine 36: 222–231.
The Lancet Infectious Diseases. For sepsis, the drugs don’t work. Lancet Infect Dis 2012, 12:89.
Hotchkiss, R.S., and I.E. Karl. 2003. The pathophysiology and treatment of sepsis. New England Journal of Medicine 348: 138–150.
Perl, M., C.S. Chung, M. Garber, X. Huang, and A. Ayala. 2006. Contribution of anti-inflammatory/immune suppressive processes to the pathology of sepsis. Frontiers in Bioscience 11: 272–299.
Ayala, A., C.S. Chung, P.S. Grutkoski, and G.Y. Song. 2003. Mechanisms of immune resolution. Critical Care Medicine 31: S558–S571.
Skrupky, L.P., P.W. Kerby, and R.S. Hotchkiss. 2011. Advances in the management of sepsis and the understanding of key immunologic defects. Anesthesiology 115: 1349–1362.
Docke, W.D., F. Randow, U. Syrbe, D. Krausch, K. Asadullah, P. Reinke, H.D. Volk, and W. Kox. 1997. Monocyte deactivation in septic patients: restoration by IFN-γ treatment. Nature Medicine 3: 678–681.
Monneret, G., F. Venet, B.J. Kullberg, and M.G. Netea. 2011. ICU-acquired immunosuppression and the risk for secondary fungal infections. Medical Mycology 49(Suppl 1): S17–S23.
Hotchkiss, R.S., K.W. Tinsley, P.E. Swanson, R.E. Schmieg, J.J. Hui Jr., K.C. Chang, D.F. Osborne, B.D. Freeman, J.P. Cobb, T.G. Buchman, and I.E. Karl. 2001. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. Journal of Immunology 166: 6952–6963.
Schefold, J.C., D. Hasper, H.D. Volk, and P. Reinke. 2008. Sepsis: time has come to focus on the later stages. Medical Hypotheses 71: 203–208.
Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392: 245–252.
Lipscomb, M.F., and B.J. Masten. 2002. Dendritic cells: immune regulators in health and disease. Physiological Reviews 82: 97–130.
Hotchkiss, R.S., K.W. Tinsley, P.E. Swanson, M.H. Grayson, D.F. Osborne, T.H. Wagner, J.P. Cobb, C. Coopersmith, and I.E. Karl. 2002. Depletion of dendritic cells, but not macrophages, in patients with sepsis. Journal of Immunology 168: 2493–2500.
Guisset, O., M.S. Dilhuydy, R. Thiébaut, J. Lefèvre, F. Camou, A. Sarrat, C. Gabinski, J.F. Moreau, and P. Blanco. 2007. Decrease in circulating dendritic cells predicts fatal outcome in septic shock. Intensive Care Medicine 33: 148–152.
Tinsley, K.W., M.H. Grayson, P.E. Swanson, A.M. Drewry, K.C. Chang, I.E. Karl, and R.S. Hotchkiss. 2003. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. Journal of Immunology 171: 909–914.
Efron, P.A., A. Martins, D. Minnich, K. Tinsley, R. Ungaro, F.R. Bahjat, R. Hotchkiss, M. Clare-Salzler, and L.L. Moldawer. 2004. Characterization of the systemic loss of dendritic cells in murine lymph nodes during polymicrobial sepsis. Journal of Immunology 173: 3035–3043.
Ding, Y., C.S. Chung, S. Newton, Y. Chen, S. Carlton, J.E. Albina, and A. Ayala. 2004. Polymicrobial sepsis induces divergent effects on splenic and peritoneal dendritic cell function in mice. Shock 22: 137–144.
Bohannon, J., W. Cui, R. Cox, R. Przkora, E. Sherwood, and T. Toliver-Kinsky. 2008. Prophylactic treatment with fms-like tyrosine kinase-3 ligand after burn injury enhances global immune responses to infection. Journal of Immunology 180: 3038–3048.
Toliver-Kinsky, T.E., W. Cui, E.D. Murphey, C. Lin, and E.R. Sherwood. 2005. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. Journal of Immunology 174: 404–410.
Gautier, E.L., T. Huby, F. Saint-Charles, B. Ouzilleau, M.J. Chapman, and P. Lesnik. 2008. Enhanced dendritic cell survival attenuates lipopolysaccharide-induced immunosuppression and increases resistance to lethal endotoxic shock. Journal of Immunology 180: 6941–6946.
Pène, F., B. Zuber, E. Courtine, C. Rousseau, F. Ouaaz, J. Toubiana, A. Tazi, J.P. Mira, and J.D. Chiche. 2008. Dendritic cells modulate lung response to Pseudomonas aeruginosa in a murine model of sepsis-induced immune dysfunction. Journal of Immunology 181: 8513–8520.
Xiang, X.S., Y.Z. Zhao, N. Li, Q.R. Li, and J.S. Li. 2010. Accumulation of DC in lamina propria induced by FMS-like tyrosine kinase 3 ligand aggravates the intestinal inflammatory response during endotoxemia. Inflammation 33: 34–45.
Samel, S., M. Keese, M. Kleczka, S. Lanig, N. Gretz, M. Hafner, J. Sturm, and S. Post. 2002. Microscopy of bacterial translocation during small bowel obstruction and ischemina in vivo—a new animal model. BMC Surgery 2: 6–9.
Almansa, R., J. Wain, E. Tamayo, D. Andaluz-Ojeda, I. Martin-Loeches, P. Ramirez, and J.F. Bermejo-Martin. 2013. Immunological monitoring to prevent and treat sepsis. Critical Care 25: 109–111.
Winter, C., K. Taut, F. Länger, M. Mack, D.E. Briles, J.C. Paton, R. Maus, M. Srivastava, T. Welte, and U.A. Maus. 2007. FMS-like tyrosine kinase 3 ligand aggravates the lung inflammatory response to Streptococcus pneumoniae infection in mice: role of dendritic cells. Journal of Immunology 179: 3099–3108.
von Wulffen, W., M. Steinmueller, S. Herold, L.M. Marsh, P. Bulau, W. Seeger, T. Welte, J. Lohmeyer, and U.A. Maus. 2007. Lung dendritic cells elicited by Fms-like tyrosine 3-kinase ligand amplify the lung inflammatory response to lipopolysaccharide. American Journal of Respiratory and Critical Care Medicine 176: 892–901.
Sun, C.M., J.A. Hall, R.B. Blank, N. Bouladoux, M. Oukka, J.R. Mora, and Y. Belkaid. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. Journal of Experimental Medicine 204: 1775–1785.
Annane, D., V. Sébille, C. Charpentier, P.E. Bollaert, B. François, J.M. Korach, G. Capellier, Y. Cohen, E. Azoulay, G. Troché, P. Chaumet-Riffaud, and E. Bellissant. 2001. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288: 862–871.
Finfer, S. 2008. Corticosteroids in septic shock. New England Journal of Medicine 358: 188–190.
Goldstein, A.L., A. Guha, M.M. Zatz, M.A. Hardy, and A. White. 1972. Purification and biological activity of thymosin, a hormone of the thymus gland. Proceedings of the National Academy of Sciences of the United States of America 69: 1800–1803.
Goldstein, A.L., and A.L. Goldstein. 2009. From lab to bedside: emerging clinical applications of thymosin alpha1. Expert Opinion on Biological Therapy 9: 593–608.
Zhang, Y., H. Chen, Y.M. Li, S.S. Zheng, Y.G. Chen, L.J. Li, L. Zhou, H.Y. Xie, and R.K. Praseedom. 2008. Thymosin alpha-1 and ulinastatin-based immunomodulatory strategy for sepsis arising from intra-abdominal infection due to carbapenem-resistant bacteria. Journal of Infectious Diseases 198: 723–730.
Conflict of Interest Statement
We declare that we have no financial or personal relationships with other people or organizations that could inappropriately influence our work, and we have no professional or personal interest of any nature in any product, service and/or company that could be construed as influencing the position presented in this manuscript entitled“Combination therapy with thymosin alpha1 and dexamethasone helps mice survive sepsis”.
Grants
This study is supported by the National Basic Research Program (973 Program) in China (no. 2007CB513005 and 2009CB522405) and the Key Project of the National Natural Science Foundation in China (30830098).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiang, Xs., Li, N., Zhao, Yz. et al. Combination Therapy with Thymosin Alpha1 and Dexamethasone Helps Mice Survive Sepsis. Inflammation 37, 402–416 (2014). https://doi.org/10.1007/s10753-013-9753-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-013-9753-5