Abstract
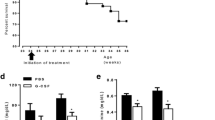
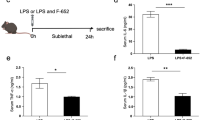
Systemic inflammatory response syndrome (SIRS) is a life-threatening disease. Recent reports have demonstrated that the immunoregulatory cells that express Gr-1, a granulocyte surface antigen, play a critical role in various pathological conditions. In the present study, we have established a mouse model of SIRS and addressed the possible contribution of Gr-1+ cells in this model. C57BL/6 mice were injected intraperitoneally with anti-Gr-1 mAb or control IgG 1 day before administration of lipopolysaccharide (LPS). All of the mice that received anti-Gr-1 mAb and LPS died early as a result of hypothermia and severe emaciation, whereas mice treated with control IgG and LPS survived the observation period. In mice treated with anti-Gr-1 mAb and LPS, acute inflammatory changes with alveolar hemorrhage were observed in the lung and proximal convoluted tubule necrosis was observed in the kidney. Serum TNF-α and IL-17A levels were markedly increased in anti-Gr-1 mAb-pretreated mice compared with those in control IgG-treated mice at 1 and 3 h after LPS administration, respectively. Flow cytometric analysis revealed an increase in TNF-α and IL-17A expression in Gr-1dull+ cells in the peripheral blood mononuclear cells. Neutralization of TNF-α by a specific mAb almost completely reversed the clinical course and inhibited the increased production of IL-17A. In addition, IL-17A KO mice were less susceptible to the lethality in this model. Thus, we established a mouse model of severe SIRS and suggested that Gr-1dull+ cells may play a critical role in the development of this pathological condition.






Similar content being viewed by others
References
Bone, R.C., R.A. Balk, F.B. Cerra, et al. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101: 1644–55.
Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420: 885–91.
Robertson, C.M., and C.M. Coopersmith. 2006. The systemic inflammatory response syndrome. Microbes and Infection 8: 1382–9.
Fink, M.P., and S.O. Heard. 1990. Laboratory models of sepsis and septic shock. Journal of Surgical Research 49: 186–96.
Takeuchi, O., and S. Akira. 2001. Toll-like receptors; their physiological role and signal transduction system. International Immunopharmacology 1: 625–35.
Salomao, R., M.K. Brunialti, M.M. Rapozo, et al. 2012. Bacterial sensing, cell signaling, and modulation of the immune response during sepsis. Shock 38: 227–42.
Egan, C.E., W. Sukhumavasi, A.L. Bierly, et al. 2008. Understanding the multiple functions of Gr-1+ cell subpopulations during microbial infection. Immunologic Research 40: 35–48.
Delano, M.J., P.O. Scumpia, J.S. Weinstein, et al. 2007. MyD88-dependent expansion of an immature GR-1+CD11b+ population induces T cell suppression and Th2 polarization in sepsis. Journal of Experimental Medicine 204: 1463–74.
Sawanobori, Y., S. Ueha, M. Kurachi, et al. 2008. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 111: 5457–66.
Bronte, V., E. Apolloni, A. Cabrelle, et al. 2000. Identification of a CD11b+/Gr-1+/CD31+ myeloid progenitor capable of activating or suppressing CD8+ T cells. Blood 96: 3838–46.
Ezernitchi, A.V., I. Vaknin, L. Cohen-Daniel, et al. 2006. TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. Journal of Immunology 177: 4763–72.
Gómez-García, L., L.M. López-Marín, R. Saavedra, et al. 2005. Intact glycans from cestode antigens are involved in innate activation of myeloid suppressor cells. Parasite Immunology 27: 395–405.
Goris, R.J.A. 1996. MODS/SIRS: Result of an overwhelming inflammatory response. World Journal of Surgery 20: 418–21.
Schlag, G., and H. Real. 1996. Mediators of injury and inflammation. World Journal of Surgery 20: 406–10.
Thomas, J.R., J.M. Harlan, C.L. Rice, et al. 1992. Role of leukocyte CD11/CD18 complex in endotoxic and septic shock in rabbits. J App Physiol 73: 1510–6.
Nakae, S., Y. Komiyama, A. Nambu, et al. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17: 375–87.
Iwakura, Y., S. Nakae, S. Saijo, et al. 2008. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunology Reviews 226: 57–79.
de Jong, H.K., T. van der Poll, and W.J. Wiersinga. 2010. The systemic pro-inflammatory response in sepsis. Journal of Innate Immunity 2: 422–30.
Buras, J.A., B. Holzmann, and M. Sitkovsky. 2005. Animal models of sepsis: Setting the stage. Nature Reviews Drug Discovery 4: 854–65.
Deitch, E.A. 1998. Animal models of sepsis and shock: A review and lessons learned. Shock 9: 1–11.
Auffray, C., M.H. Sieweke, and F. Geissmann. 2009. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annual Review of Immunology 27: 669–92.
Serbina, N.V., T. Jia, T. Hohl, et al. 2008. Monocyte-mediated defense against microbial pathogens. Annual Review of Immunology 26: 421–52.
Sunderkotter, C., T. Nikolic, M.J. Dillon, et al. 2004. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. Journal of Immunology 172: 4410–7.
Palframan, R.T., S. Jung, G. Cheng, et al. 2001. Inflammatory chemokine transport and presentation in HEV: A remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. Journal of Experimental Medicine 194: 1361–73.
Aoyagi, T., N. Yamamoto, M. Hatta, et al. 2011. Activation of pulmonary invariant NKT cells leads to exacerbation of acute lung injury caused by LPS through local production of IFN-γ and TNF-α by Gr-1+ monocytes. International Immunology 23: 97–108.
Deitch, E.A. 1995. Tumor necrosis factor as the proximal mediator of sepsis: Or this too will pass. Critical Care Medicine 23: 1457–8.
Bone, R.C. 1996. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: What we do and do not know about cytokine regulation. Critical Care Medicine 24: 163–72.
Beutler, B., I.W. Milsark, and A.C. Cerami. 1985. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 229: 869–71.
Tracey, K.J., Y. Fong, D.G. Hesse, et al. 1987. Anti-cachectin/TNF-α monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330: 662–4.
Hinshaw, L.B., T.E. Emerson Jr., F.B. Taylor Jr., et al. 1992. Lethal Staphylococcus aureus-induced shock in primates: Prevention of death with anti-TNF-α antibody. Journal of Trauma 33: 568–73.
Weaver, C.T., R.D. Hatton, P.R. Mangan, et al. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual Review of Immunology 25: 821–52.
Flierl, M.A., D. Rittirsch, H. Gao, et al. 2008. Adverse functions of IL-17A in experimental sepsis. FASEB Journal 22: 2198–205.
Bosmann, M., J.V. Sarma, G. Atefi, et al. 2012. Evidence for anti-inflammatory effects of C5a on the innate IL-17A/IL-23 axis. FASEB Journal 26: 1640–51.
Ogiku, M., H. Kono, M. Hara, et al. 2012. Interleukin-17A plays a pivotal role in polymicrobial sepsis according to studies using IL-17A knockout mice. Journal of Surgical Research 174: 142–9.
Sutton, C.E., L.A. Mielke, and K.H. Mills. 2012. IL-17-producing γδ T cells and innate lymphoid cells. European Journal of Immunology 42: 2221–31.
Passos, S.T., J.S. Silver, A.C. O'Hara, et al. 2010. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. Journal of Immunology 184: 1776–1783.
Michel, M.L., A.C. Keller, C. Paget, et al. 2007. Identification of an IL-17-producing NK1.1− iNKT cell population involved in airway neutrophilia. Journal of Experimental Medicine 204: 995–1001.
Rachitskaya, A.V., A.M. Hansen, R. Horai, et al. 2008. Cutting edge: NKT cells constitutively express IL-23 receptor and RORγt and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. Journal of Immunology 180: 5167–71.
Bosmann, M., and P.A. Ward. 2012. Therapeutic potential of targeting IL-17 and IL-23 in sepsis. Clin Transl Med 1: 4–5.
Gu, Y., J. Yang, X. Ouyang, et al. 2008. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. European Journal of Immunology 38: 1807–13.
Umemura, M., A. Yahagi, S. Hamada, et al. 2007. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette–Guerin infection. Journal of Immunology 178: 3786–96.
Ouyang, W., S. Rutz, N.K. Crellin, et al. 2011. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annual Review of Immunology 29: 71–109.
Li, M.O., Y.Y. Wan, S. Sanjabi, et al. 2006. Transforming growth factor-beta regulation of immune responses. Annual Review of Immunology 24: 99–146.
Cavaillon, J.M., and M. Adib-Conquy. 2006. Bench-to-bedside review: Endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Critical Care 10: 233–40.
St. John, R.C., and P.M. Dorinsky. 1993. Immunologic therapy for ARDS, septic shock, and multiple-organ failure. Chest 103: 932–43.
Acknowledgments
The authors thank Dr. Fujiro Sendo (Yamagata University, Yamagata, Japan) and Dr. Akio Nakane (Hirosaki University Graduate School of Medicine, Hirosaki, Japan) for their kind gifts of anti-Gr-1 mAb and anti-TNF-α mAb, respectively. This work was supported in part by a Grant from the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Challenging Exploratory Research (23659841)) and a Grant from the Ministry of Health, Labor and Welfare of Japan (Research on Emerging and Re-emerging Infectious Diseases; 22-SHINKOU-IPPAN-014).
Conflict of interest
The authors declare that they have no financial conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Tanno and Y. Akahori contributed equally to this study.
ELECTRONIC SUPPLEMENTARY MATERIAL
Below is the link to the electronic supplementary material.
Fig. S1
Effect of anti-Gr-1 mAb on the expression of Gr-1 on PBMC. Mice were treated with rat IgG or anti-Gr-1 mAb, and 24 h later, PBMC were stained with anti-Gr-1 mAb and analyzed using a flow cytometry. Dotted line, rat IgG-treated; shaded area, anti-Gr-1 mAb-treated. (PPT 82 kb)
Rights and permissions
About this article
Cite this article
Tanno, D., Akahori, Y., Toyama, M. et al. Involvement of Gr-1dull+ Cells in the Production of TNF-α and IL-17 and Exacerbated Systemic Inflammatory Response Caused by Lipopolysaccharide. Inflammation 37, 186–195 (2014). https://doi.org/10.1007/s10753-013-9729-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-013-9729-5