Abstract
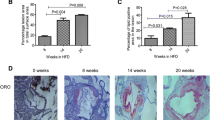
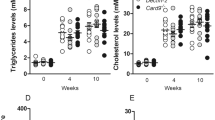
The objective of this study was to investigate the effect of hyperlipidemia (HLP) on innate immune responses to lipopolysaccharide (LPS). Male New Zealand white rabbits were fed a normal diet (ND) or a high-fat diet (HFD) for 8 weeks. In vivo, the rabbits were injected intravenously with LPS for 24 h. In vitro, peripheral mononuclear cells were collected and stimulated (or unstimulated) with LPS for 24 h. Assay results were analyzed with one-way ANOVA or an equivalent non-parametric test. A P value of 0.05 was considered statistically significant. Despite having no influence in body weight, the HFD intake significantly increased serum lipids, C-reactive protein (CRP), nuclear factor (NF)-κB subunit p65, Toll-like receptor (TLR)-4, SR-A and FAS. Although we found increased circulating tumor necrosis factor (TNF)-α, interleukin (IL)-6, CRP, IL-1β, and IL-10 in the ND-fed rabbits, no significant difference was found in the LPS-stimulated production of TNF-α, IL-6, and CRP in the HFD-fed rabbits. The macrophages harvested from the HFD-fed rabbits developed a blunted inflammatory response, with lower mRNA expression of TNF-α, IL-6, CRP, TLR-4, SR-A, FAS, and Bcl-2 than that expressed by the ND group. In the HFD-fed animals, LPS incubation decreased NF-κB subunit p65 expression, whereas the cytoplasmic phosphorylation of the inhibitor of NF-κB protein was enhanced. These data indicate that HLP displayed a form of innate immune paralysis, including reduced pro- and anti-inflammatory cytokine release to external stimulus, which was related to the altered TLR-NF-κB signaling pathway and altered pro- and anti-apoptotic processes in macrophages.






Similar content being viewed by others
References
Cameron, A.J., J.E. Shaw, and P.Z. Zimmet. 2004. The metabolic syndrome: prevalence in worldwide populations. Endocrinology and Metabolism Clinics of North America 33: 351–375. table of contents.
Kassi, E., P. Pervanidou, G. Kaltsas, and G. Chrousos. 2011. Metabolic syndrome: definitions and controversies. BMC Medicine 9: 48.
Wellen, K.E., and G.S. Hotamisligil. 2003. Obesity-induced inflammatory changes in adipose tissue. The Journal of Clinical Investigation 112: 1785–1788.
Lawrence, C.B., D. Brough, and E.M. Knight. 2012. Obese mice exhibit an altered behavioural and inflammatory response to lipopolysaccharide. Disease Models & Mechanisms 5: 649–659.
Yang, Z.H., H. Miyahara, J. Takeo, and M. Katayama. 2012. Diet high in fat and sucrose induces rapid onset of obesity-related metabolic syndrome partly through rapid response of genes involved in lipogenesis, insulin signalling and inflammation in mice. Diabetology and Metabolic Syndrome 4: 32.
Ghanim, H., S. Abuaysheh, C.L. Sia, et al. 2009. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care 32: 2281–2287.
Huang, H., T. Liu, J.L. Rose, R.L. Stevens, and D.G. Hoyt. 2007. Sensitivity of mice to lipopolysaccharide is increased by a high saturated fat and cholesterol diet. J Inflamm (Lond) 4: 22.
Kim, M.S., M.S. Choi, and S.N. Han. 2011. High fat diet-induced obesity leads to proinflammatory response associated with higher expression of NOD2 protein. Nutrition Research Practice 5: 219–223.
Borges, M.C., Vinolo, M.A., Crisma, A.R., et al. 2012. High-fat diet blunts activation of the nuclear factor-kappaB signaling pathway in lipopolysaccharide-stimulated peritoneal macrophages of Wistar rats. LID - 10.1016/j.nut.2012.06.008 [doi]. Nutrition.
Janssens, S., and R. Beyaert. 2003. Role of Toll-like receptors in pathogen recognition. Clinical Microbiology Reviews 16: 637–646.
Xu, X.H., P.K. Shah, E. Faure, et al. 2001. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104: 3103–3108.
Shi, H., M.V. Kokoeva, K. Inouye, I. Tzameli, H. Yin, and J.S. Flier. 2006. TLR4 links innate immunity and fatty acid-induced insulin resistance. The Journal of Clinical Investigation 116: 3015–3025.
Kerttula, Y., M. Vaara, L. Pyhala, H. Sariola, E. Kostiainen, and J.K. Huttunen. 1986. Effect of bacterial lipopolysaccharide on serum lipids and on the development of aortic atherosclerosis in rabbits. Atherosclerosis 59: 307–312.
Ebersole, J.L., J. Stevens, M.J. Steffen, I.D. Dawson, and M.J. Novak. 2010. Systemic endotoxin levels in chronic indolent periodontal infections. Journal of Periodontal Research 45: 1–7.
de Almeida, M.C., A.C. Silva, A. Barral, and N.M. Barral. 2000. A simple method for human peripheral blood monocyte isolation. Memórias do Instituto Oswaldo Cruz 95: 221–223.
Martin, M.J., S.B. Hulley, W.S. Browner, L.H. Kuller, and D. Wentworth. 1986. Serum cholesterol, blood pressure, and mortality: implications from a cohort of 361,662 men. Lancet 2: 933–936.
Assmann, G., H. Schulte, E.A. Von, and Y. Huang. 1996. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis 124: 11–20.
Austin, M.A., J.E. Hokanson, and K.L. Edwards. 1998. Hypertriglyceridemia as a cardiovascular risk factor. The American Journal of Cardiology 81: 7B–12B.
Zhang, L., W.H. Zhang, L. Zhang, and P.Y. Wang. 2011. Prevalence of overweight/obesity and its associations with hypertension, diabetes, dyslipidemia, and metabolic syndrome: a survey in the suburban area of Beijing, 2007. Obesity Facts 4: 284–289.
Safwat, G.M., S. Pisano, E. D’Amore, et al. 2009. Induction of non-alcoholic fatty liver disease and insulin resistance by feeding a high-fat diet in rats: does coenzyme Q monomethyl ether have a modulatory effect. Nutrition 25: 1157–1168.
Kolovou, G., K. Anagnostopoulou, D.P. Mikhailidis, and D.V. Cokkinos. 2008. Apolipoprotein E knockout models. Current Pharmaceutical Design 14: 338–351.
Kamimura, R., S. Suzuki, H. Sakamoto, N. Miura, K. Misumi, and K. Miyahara. 1999. Development of atherosclerotic lesions in cholesterol-loaded rabbits. Experimental Animals 48: 1–7.
Kolodgie, F.D., A.S. Katocs Jr., E.E. Largis, et al. 1996. Hypercholesterolemia in the rabbit induced by feeding graded amounts of low-level cholesterol. Methodological considerations regarding individual variability in response to dietary cholesterol and development of lesion type. Arteriosclerosis, Thrombosis, and Vascular Biology 16: 1454–1464.
Yanni, A.E. 2004. The laboratory rabbit: an animal model of atherosclerosis research. Laboratory Animals 38: 246–256.
Arner, P., S. Bernard, M. Salehpour, et al. 2011. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature 478: 110–113.
Franssen, R., H. Monajemi, E.S. Stroes, and J.J. Kastelein. 2011. Obesity and dyslipidemia. The Medical Clinics of North America 95: 893–902.
Amar, S., Q. Zhou, Y. Shaik-Dasthagirisaheb, and S. Leeman. 2007. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proceedings of the National Academy of Sciences of the United States of America 104: 20466–20471.
Cousin, B., M. Andre, L. Casteilla, and L. Penicaud. 2001. Altered macrophage-like functions of preadipocytes in inflammation and genetic obesity. Journal of Cellular Physiology 186: 380–386.
Ross, R. 1999. Atherosclerosis—an inflammatory disease. The New England Journal of Medicine 340: 115–126.
Libby, P. 2002. Inflammation in atherosclerosis. Nature 420: 868–874.
Li, Q., and I.M. Verma. 2002. NF-kappaB regulation in the immune system. Nature Reviews Immunology 2: 725–734.
Baker, R.G., M.S. Hayden, and S. Ghosh. 2011. NF-kappaB, inflammation, and metabolic disease. Cell Metabolism 13: 11–22.
Zhou, Q., S.E. Leeman, and S. Amar. 2009. Signaling mechanisms involved in altered function of macrophages from diet-induced obese mice affect immune responses. Proceedings of the National Academy of Sciences of the United States of America 106: 10740–10745.
Zhou, Q., S.E. Leeman, and S. Amar. 2011. Signaling mechanisms in the restoration of impaired immune function due to diet-induced obesity. Proceedings of the National Academy of Sciences of the United States of America 108: 2867–2872.
Yu, H., T. Ha, L. Liu, et al. 2012. Scavenger receptor A (SR-A) is required for LPS-induced TLR4 mediated NF-kappaB activation in macrophages. Biochimica et Biophysica Acta 1823: 1192–1198.
Seimon, T.A., A. Obstfeld, K.J. Moore, D.T. Golenbock, and I. Tabas. 2006. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proceedings of the National Academy of Sciences of the United States of America 103: 19794–19799.
Amiel, E., A. Alonso, S. Uematsu, S. Akira, M.E. Poynter, and B. Berwin. 2009. Pivotal advance: toll-like receptor regulation of scavenger receptor-A-mediated phagocytosis. Journal of Leukocyte Biology 85: 595–605.
Xu, W.Y., L. Wang, H.M. Wang, et al. 2007. TLR2 and TLR4 agonists synergistically up-regulate SR-A in RAW264.7 through p38. Molecular Immunology 44: 2315–2323.
Cotena, A., S. Gordon, and N. Platt. 2004. The class A macrophage scavenger receptor attenuates CXC chemokine production and the early infiltration of neutrophils in sterile peritonitis. Journal of Immunology 173: 6427–6432.
Chen, Y., F. Wermeling, J. Sundqvist, et al. 2010. A regulatory role for macrophage class A scavenger receptors in TLR4-mediated LPS responses. European Journal of Immunology 40: 1451–1460.
Ben-Hur, H., P. Gurevich, A. Ben-Arie, et al. 2000. Apoptosis and apoptosis-related proteins (Fas, Fas ligand, bcl-2, p53) in macrophages of human ovarian epithelial tumors. European Journal of Gynaecological Oncology 21: 141–145.
Van Parijs, L., D.A. Peterson, and A.K. Abbas. 2009. Retraction. The Fas/Fas ligand pathway and Bcl-2 regulate T cell responses to model self and foreign antigens. Immunity 30: 611.
Liang, C.Z., J.K. Zhang, Z. Shi, B. Liu, C.Q. Shen, and H.M. Tao. 2012. Matrine induces caspase-dependent apoptosis in human osteosarcoma cells in vitro and in vivo through the upregulation of Bax and Fas/FasL and downregulation of Bcl-2. Cancer Chemotherapy and Pharmacology 69: 317–331.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (Grant No. 81170973, 30973326). We sincerely thank the members of our laboratory for critical comments and discussion, and Comparative Medicine Facility of Fuzhou General Hospital of Nanjing Military Region for assistance with animal experiments.
The authors have no conflicts of interest to disclose, whether financial or of any other nature.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, S., Lin, G., Lei, L. et al. Hyperlipidemia Modifies Innate Immune Responses to Lipopolysaccharide via the TLR-NF-κB Signaling Pathway. Inflammation 36, 968–976 (2013). https://doi.org/10.1007/s10753-013-9628-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-013-9628-9