Abstract
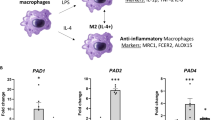
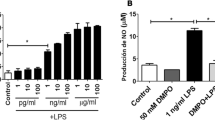
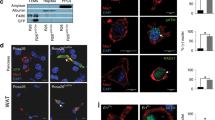
The copper transporter ATP7A has attracted significant attention since the discovery of its gene mutation leading to human Menkes disease. We previously reported that ATP7A is highly expressed in the human vasculature and identified a novel vascular function of ATP7A in modulation of the expression and activity of extracellular superoxide dismutase. We recently identified that ATP7A expression in THP-1 cells (a monocyte/macrophage model cell line) plays a role in the oxidation of low density lipoproteins, indicating that it is necessary to further investigate its expression and function in monocytes/macrophages. In the current study, we demonstrated the protein and mRNA expression of ATP7A in human peripheral blood mononuclear cell (PBMC)-derived macrophages and alveolar macrophages. ATP7A was strongly co-localized with the trans-Golgi apparatus in PBMC-derived macrophages. Intracellular copper, detected by synchrotron X-ray fluorescence microscopy, was found to be distributed to the nucleus and cytoplasm in human THP-1 cells. To confirm the role of endogenous ATP7A in macrophage copper homeostasis, we performed inductively coupled plasma mass spectrometry in murine peritoneal macrophages, which showed markedly increased intracellular copper levels in macrophages isolated from ATP7A-deficient mice versus control mice. Moreover, the role of ATP7A in regulating macrophage responses to dermal wounds was studied by introduction of control and ATP7A-downregulated THP-1 cells into dermal wounds of nude mice. Infiltration of THP-1 cells into the wounded area (detected by expression of human macrophage markers MAC2 and CD68) was reduced in response to downregulation of ATP7A, hinting decreased macrophage accumulation subsequent to dermal wounds. In summary, alongside our previous studies, these findings indicate that human macrophage ATP7A is localized in the trans-Golgi apparatus, regulates intracellular copper levels, and mediates macrophage responses to a dermal wound.






Similar content being viewed by others
Abbreviations
- calcein AM:
-
Calcein acetoxymethyl ester
- cPLA2α :
-
Cytosolic phospholipase A2α
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DIC:
-
Differential interference contrast
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- PBMC:
-
Peripheral blood mononuclear cell
- PBS:
-
Phosphate-buffered saline
- PDGF:
-
Platelet-derived growth factor
- PMA:
-
Phorbol-12-myristate-13-acetate
- SXRF:
-
Synchrotron X-ray fluorescence
- VEGF:
-
Vascular endothelial growth factor
- VSMC:
-
Vascular smooth muscle cell
References
Easter, R.N., et al. 2010. Vascular metallomics: copper in the vasculature. Vascular Medicine 15(1): 61–69.
Mertz, W. 1981. The essential trace elements. Science 213(4514): 1332–1338.
Rae, T.D., et al. 1999. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284(5415): 805–808.
Chelly, J., et al. 1993. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nature Genetics 3(1): 14–19.
Levinson, B., et al. 1994. The mottled gene is the mouse homologue of the Menkes disease gene. Nature Genetics 6(4): 369–373.
Mercer, J.F., et al. 1993. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nature Genetics 3(1): 20–25.
Qin, Z., et al. 2006. Essential role for the Menkes ATPase in activation of extracellular superoxide dismutase: implication for vascular oxidative stress. The FASEB Journal 20(2): 334–336.
Qin, Z., et al. 2008. Role of Menkes ATPase in angiotensin ii-induced hypertension. A key modulator for extracellular superoxide dismutase function. Hypertension 52: 945.
Babu, U., and M.L. Failla. 1990. Respiratory burst and candidacidal activity of peritoneal macrophages are impaired in copper-deficient rats. The Journal of Nutrition 120(12): 1692–1699.
Jones, D.G., and N.F. Suttle. 1983. The effect of copper deficiency on the resistance of mice to infection with Pasteurella haemolytica. Journal of Comparative Pathology 93(1): 143–149.
White, C., et al. 2009. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. The Journal of Biological Chemistry 284(49): 33949–33956.
White, C., et al. 2009. Copper transport into the secretory pathway is regulated by oxygen in macrophages. Journal of Cell Science 122(Pt 9): 1315–1321.
Zheng, Z., et al. 2010. Altered microglial copper homeostasis in a mouse model of Alzheimer's disease. Journal of Neurochemistry 114(6): 1630–1638.
Afton, S., et al. 2009. Copper egress is induced by PMA in human THP-1 monocytic cell line. Biometals 22(3): 531–539.
Qin, Z., et al. 2010. Participation of ATP7A in macrophage mediated oxidation of LDL. Journal of Lipid Research 51(6): 1471–1477.
Finney, L., et al. 2007. X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. Proceedings of the National Academy of Sciences of the United States of America 104(7): 2247–2252.
Yang, L., et al. 2005. Imaging of the intracellular topography of copper with a fluorescent sensor and by synchrotron X-ray fluorescence microscopy. Proceedings of the National Academy of Sciences of the United States of America 102(32): 11179–11184.
Afton, S., et al. 2009. Copper egress is induced by PMA in human THP-1 cells: a potential regulator of VEGFR1. Biometals 22(3): 9.
Qin, Z., et al. 2011. Quantitative trace metal imaging with high spatial resolution: applications in biomedicine. Metallomics 3(1):28–37
Becker, J.S., et al. 2010. Bioimaging of metals and biomolecules in mouse heart by laser ablation inductively coupled plasma mass spectrometry and secondary ion mass spectrometry. Anal Chem 82: 9528–33.
Kemner, K.M., et al. 2004. Elemental and redox analysis of single bacterial cells by X-ray microbeam analysis. Science 306(5696): 686–687.
Ralle, M., et al. 2010. Wilson disease at a single cell level: intracellular copper trafficking activates compartment-specific responses in hepatocytes. The Journal of Biological Chemistry 285(40): 30875–30883.
Collins, J.F., et al. 2009. Alternative splicing of the Menkes copper ATPase (Atp7a) transcript in the rat intestinal epithelium. American Journal of Physiology—Gastrointestinal and Liver Physiology 297: G695–707.
Ashino, T., et al. 2010. Unexpected role of the copper transporter ATP7A in PDGF-induced vascular smooth muscle cell migration. Circ Res 107: 787–799.
Rees, R.S., B.F. Adamson, and W.J. Lindblad. 2001. Use of a cell-based interactive wound dressing to enhance healing of excisional wounds in nude mice. Wound Repair and Regeneration 9(4): 297–304.
Manuel, J.A., and B. Gawronska-Kozak. 2006. Matrix metalloproteinase 9 (MMP-9) is upregulated during scarless wound healing in athymic nude mice. Matrix Biology 25(8): 505–514.
Borkow, G., J. Gabbay, and R.C. Zatcoff. 2008. Could chronic wounds not heal due to too low local copper levels? Medical Hypotheses 70(3): 610–613.
Liusuwan, R.A., et al. 2008. Impaired healing because of copper deficiency in a pediatric burn patient: a case report. The Journal of Trauma 65(2): 464–466.
Chavakis, T., et al. 1999. Molecular mechanisms of zinc-dependent leukocyte adhesion involving the urokinase receptor and beta2-integrins. Blood 93(9): 2976–2983.
Wells, C.M., et al. 2004. Rac1-deficient macrophages exhibit defects in cell spreading and membrane ruffling but not migration. Journal of Cell Science 117(Pt 7): 1259–1268.
Ronald, J.A., et al. 2001. Differential regulation of transendothelial migration of THP-1 cells by ICAM-1/LFA-1 and VCAM-1/VLA-4. Journal of Leukocyte Biology 70(4): 601–609.
Patel, T.R., and S.A. Corbett. 2004. Simvastatin suppresses LPS-induced Akt phosphorylation in the human monocyte cell line THP-1. The Journal of Surgical Research 116(1): 116–120.
Capo, C., et al. 1998. Effect of cytotoxic necrotizing factor-1 on actin cytoskeleton in human monocytes: role in the regulation of integrin-dependent phagocytosis. Journal of Immunology 161(8): 4301–4308.
Wey, J.S., et al. 2005. Vascular endothelial growth factor receptor-1 promotes migration and invasion in pancreatic carcinoma cell lines. Cancer 104(2): 427–438.
Fan, F., et al. 2005. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene 24(16): 2647–2653.
Yang, A.D., et al. 2006. Vascular endothelial growth factor receptor-1 activation mediates epithelial to mesenchymal transition in human pancreatic carcinoma cells. Cancer Research 66(1): 46–51.
Zhou, Y., K. Bourcy, and Y.J. Kang. 2009. Copper-induced regression of cardiomyocyte hypertrophy is associated with enhanced vascular endothelial growth factor receptor-1 signalling pathway. Cardiovascular Research 84(1): 54–63.
Akuzawa, N., et al. 2000. Zinc finger transcription factor Egr-1 activates Flt-1 gene expression in THP-1 cells on induction for macrophage differentiation. Arteriosclerosis, Thrombosis, and Vascular Biology 20(2): 377–384.
Worley, J.R., et al. 2003. Metalloproteinase expression in PMA-stimulated THP-1 cells. Effects of peroxisome proliferator-activated receptor-gamma (PPAR gamma) agonists and 9-cis-retinoic acid. The Journal of Biological Chemistry 278(51): 51340–51346.
Tuomisto, T.T., et al. 2005. Analysis of gene and protein expression during monocyte-macrophage differentiation and cholesterol loading—cDNA and protein array study. Atherosclerosis 180(2): 283–291.
Zhu, X., et al. 1999. Cytosolic phospholipase A2 activation is essential for beta 1 and beta 2 integrin-dependent adhesion of human eosinophils. Journal of Immunology 163(6): 3423–3429.
Myou, S., et al. 2001. Blockade of eosinophil migration and airway hyperresponsiveness by cPLA2-inhibition. Nature Immunology 2(2): 145–149.
Neeli, I., et al. 2004. An essential role of the Jak-2/STAT-3/cytosolic phospholipase A(2) axis in platelet-derived growth factor BB-induced vascular smooth muscle cell motility. The Journal of Biological Chemistry 279(44): 46122–46128.
Maulik, N. 2002. Redox signaling of angiogenesis. Antioxidants Redox Signaling 4(5): 805–815.
Tojo, T., et al. 2005. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation 111(18): 2347–2355.
Sen, C.K. 2003. The general case for redox control of wound repair. Wound Repair and Regeneration 11(6): 431–438.
Kim, H.W., et al. 2007. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circulation Research 101(4): 409–419.
Acknowledgments
This work was supported by a National Scientist Development Grant (0835268N) from the American Heart Association and grants HL-076684 and HL-62984 from the National Institute of Health. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. We thank Birgit Ehmer and Chet Closson for assisting with the immunofluorescence microscopy and confocal microscopy, Dr. Oyebode Olakanmi for developing the protocol to isolate human PBMC-derived macrophages, and Dr. Dennis McGraw for providing human alveolar macrophages.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, H.W., Chan, Q., Afton, S.E. et al. Human Macrophage ATP7A is Localized in the trans-Golgi Apparatus, Controls Intracellular Copper Levels, and Mediates Macrophage Responses to Dermal Wounds. Inflammation 35, 167–175 (2012). https://doi.org/10.1007/s10753-011-9302-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-011-9302-z