Abstract
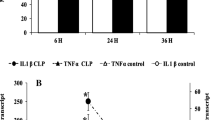
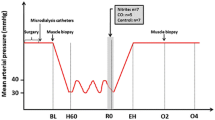
The objective of this study was to investigate early effects of peritoneal inflammation on the mitochondrial function in the vital organs, liver and kidney, and their relation to inflammatory and oxidative stress mediators. The study was performed on 14 domestic pigs. Peritoneal inflammation was induced in anesthetized pigs after a midline laparotomy by autologous feces. Fluid resuscitation maintained a MAP above 60 mmHg. Animals were sacrificed 12 h later, and tissue samples were obtained to determine mitochondrial function, mRNA levels of relevant genes [inducible NO synthase (iNOS), inducible HO (HO-1), tumor necrosis factor-alpha (TNF-alpha)], generation of reactive oxygen species (ROS), and HO-1 activity. We found impaired mitochondrial function in both liver and kidney, based on decreased state 3 respiration in the liver and increased states 2 and 4 respiration in the kidney at 12 h. This was accompanied by increased TNF-alpha protein in the blood and up-regulation of TNF-alpha mRNA in the liver. Free iron was elevated in the liver but not in the kidney. In the kidney, mitochondrial ROS production was increased. Nitric oxide levels in blood remained unchanged, corresponding to unchanged levels of iNOS mRNA expression in liver and kidney. Similarly, HO-1 mRNA and heme oxygenase (HO)-activity were unchanged. The inflammatory response in the absence of characteristic septic symptoms was not associated with morphological organ damage at this early time point. Peritoneal inflammation in pigs caused mitochondrial dysfunction in liver and kidney, preceding signs of organ damage. We did not find proof that mitochondrial dysfunction was due to increased levels of either nitric oxide (NO) or products of HO, but it was accompanied by increased levels of oxidative stress markers.






Similar content being viewed by others
References
Geller, E.R., S. Jankauskas, and J. Kirkpatrick. 1986. Mitochondrial death in sepsis: a failed concept. Journal of Surgical Research 40: 514–517.
Mela-Riker, L., D. Bartos, A.A. Vlessis, L. Widener, P. Muller, and D.D. Trunkey. 1992. Chronic hyperdynamic sepsis in the rat. II. Characterization of liver and muscle energy metabolism. Circulatory Shock 36: 83–92.
Taylor, D.E., A.J. Ghio, and C.A. Piantadosi. 1995. Reactive oxygen species produced by liver mitochondria of rats in sepsis. Archives of Biochemistry and Biophysics 316: 70–76.
Mela, L., L.V. Bacalzo Jr., and L.D. Miller. 1971. Defective oxidative metabolism of rat liver mitochondria in hemorrhagic and endotoxin shock. American Journal of Physiology 220: 571–577.
Kantrow, S.P., D.E. Taylor, M.S. Carraway, and C.A. Piantadosi. 1997. Oxidative metabolism in rat hepatocytes and mitochondria during sepsis. Archives of Biochemistry and Biophysics 345: 278–288.
Crouser, E.D., M.W. Julian, J.E. Huff, M.S. Joshi, J.A. Bauer, M.E. Gadd, M.D. Wewers, and D.R. Pfeiffer. 2004. Abnormal permeability of inner and outer mitochondrial membranes contributes independently to mitochondrial dysfunction in the liver during acute endotoxemia. Critical Care Medicine 32: 478–488.
Crouser, E.D., M.W. Julian, D.V. Blaho, and D.R. Pfeiffer. 2002. Endotoxin-induced mitochondrial damage correlates with impaired respiratory activity. Critical Care Medicine 30: 276–284.
Tanaka, J., Y. Kono, Y. Shimahara, T. Sato, R.T. Jones, R.A. Cowley, and B.F. Trump. 1982. A study of oxidative phosphorylative activity and calcium-induced respiration of rat liver mitochondria following living Escherichia coli injection. Advances in Shock Research 7: 77–90.
Taylor, D.E., S.P. Kantrow, and C.A. Piantadosi. 1998. Mitochondrial respiration after sepsis and prolonged hypoxia. American Journal of Physiology 275: L139–L144.
Lu, S.M., S.M. Song, J.C. Liu, H.M. Yang, P. Li, and Z.G. Wang. 2003. Changes of proton transportation across the inner mitochondrial membrane and H+-ATPase in endotoxic shock rats. Chin J Traumatol 6: 292–296.
Garrison, R.N., D.J. Ratcliffe, and D.E. Fry. 1982. The effects of peritonitis on murine renal mitochondria. Advances in Shock Research 7: 71–76.
Clemens, M., I.H. Chaudry, and A.E. Baue. 1981. Oxidative capability of hepatic tissue in late sepsis. Advances in Shock Research 6: 55–64.
Townsend, M.C., M.W. Gauderer, M.D. Yokum, and D.E. Fry. 1986. Alterations of hepatic mitochondrial function in a model of peritonitis in immature rats. Journal of Pediatric Surgery 21: 521–524.
Hirai, F., H. Aoyama, M. Ohtoshi, S. Kawashima, K. Ozawa, and T. Tobe. 1984. Significance of mitochondrial enhancement in hepatic energy metabolism in relation to alterations in hemodynamics in septic pigs with severe peritonitis. European Surgical Research 16: 148–155.
Porta, F., J. Takala, C. Weikert, H. Bracht, A. Kolarova, B.H. Lauterburg, E. Borotto, and S.M. Jakob. 2006. Effects of prolonged endotoxemia on liver, skeletal muscle and kidney mitochondrial function. Crit Care 10: R118.
Trager, K., P. Radermacher, K.M. Rieger, A. Vlatten, J. Vogt, T. Iber, J. Adler, U. Wachter, R. Grover, M. Georgieff, and B. Santak. 1999. Norepinephrine and nomega-monomethyl-l-arginine in porcine septic shock: effects on hepatic O2 exchange and energy balance. American Journal of Respiratory and Critical Care Medicine 159: 1758–1765.
Mikhal'chik, E.V., S.M. Titkova, M.V. Anurov, A.P. Ettinger, and L.G. Korkina. 2006. Protective effect of complex antioxidant preparation containing vitamins and amino acids in rats with burn trauma complicated by endotoxemia. Bulletin of Experimental Biology and Medicine 141: 688–690.
Cadenas, S., and A.M. Cadenas. 2002. Fighting the stranger-antioxidant protection against endotoxin toxicity. Toxicology 180: 45–63.
Shen, K.P., Y.C. Lo, R.C. Yang, H.W. Liu, I.J. Chen, and B.N. Wu. 2005. Antioxidant eugenosedin-A protects against lipopolysaccharide-induced hypotension, hyperglycaemia and cytokine immunoreactivity in rats and mice. Journal of Pharmacy and Pharmacology 57: 117–125.
Luyendyk, J.P., J.D. Piper, M. Tencati, K.V. Reddy, T. Holscher, R. Zhang, J. Luchoomun, X. Chen, W. Min, C. Kunsch, and N. Mackman. 2007. A novel class of antioxidants inhibit LPS induction of tissue factor by selective inhibition of the activation of ASK1 and MAP kinases. Arteriosclerosis, Thrombosis, and Vascular Biology 27: 1857–1863.
Victor, V.M., M. Rocha, J.V. Esplugues, and F.M. De la. 2005. Role of free radicals in sepsis: antioxidant therapy. Current Pharmaceutical Design 11: 3141–3158.
Brown, G.C. 1997. Nitric oxide inhibition of cytochrome oxidase and mitochondrial respiration: implications for inflammatory, neurodegenerative and ischaemic pathologies 8. Molecular and Cellular Biochemistry 174: 189–192.
Borutaite, V., A. Matthias, H. Harris, S. Moncada, and G.C. Brown. 2001. Reversible inhibition of cellular respiration by nitric oxide in vascular inflammation. American Journal of Physiology. Heart and Circulatory Physiology 281: H2256–H2260.
Brown, G.C. 2007. Nitric oxide and mitochondria. Frontiers in Bioscience 12: 1024–1033.
Sandouka, A., E. Balogun, R. Foresti, B.E. Mann, T.R. Johnson, Y. Tayem, C.J. Green, B. Fuller, and R. Motterlini. 2005. Carbon monoxide-releasing molecules (CO-RMs) modulate respiration in isolated mitochondria. Cellular and Molecular Biology (Noisy-le-grand) 51: 425–432.
Duvigneau, J.C., C. Piskernik, S. Haindl, B. Kloesch, R.T. Hartl, M. Huttemann, I. Lee, T. Ebel, R. Moldzio, M. Gemeiner, H. Redl, and A.V. Kozlov. 2008. A novel endotoxin-induced pathway: upregulation of heme oxygenase 1, accumulation of free iron, and free iron-mediated mitochondrial dysfunction. Laboratory Investigation 88: 70–77.
Dikalov, S., M. Skatchkov, and E. Bassenge. 1997. Spin trapping of superoxide radicals and peroxynitrite by 1-hydroxy-3-carboxy-pyrrolidine and 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine and the stability of corresponding nitroxyl radicals towards biological reductants. Biochemical and Biophysical Research Communications 231: 701–704.
Dikalov, S., M. Skatchkov, and E. Bassenge. 1997. Quantification of peroxynitrite, superoxide, and peroxyl radicals by a new spin trap hydroxylamine 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine. Biochemical and Biophysical Research Communications 230: 54–57.
Kozlov, A.V., D.Y. Yegorov, Y.A. Vladimirov, and O.A. Azizova. 1992. Intracellular free iron in liver tissue and liver homogenate: studies with electron paramagnetic resonance on the formation of paramagnetic complexes with desferal and nitric oxide. Free Radical Biology & Medicine 13: 9–16.
Vanin, A.F., and A.G. Chetverikov. 1968. Paramagnetic nitrosyl complexes of heme and nonheme iron. Biofizika 13: 608–615.
McNally, S.J., J.A. Ross, G.O. James, and S.J. Wigmore. 2004. Optimization of the paired enzyme assay for heme oxygenase activity. Analytical Biochemistry 332: 398–400.
Duvigneau, J.C., R.T. Hartl, M. Teinfalt, and M. Gemeiner. 2003. Delay in processing porcine whole blood affects cytokine expression 6. Journal of Immunological Methods 272: 11–21.
Duvigneau, J.C., R.T. Hartl, S. Groiss, and M. Gemeiner. 2005. Quantitative simultaneous multiplex real-time PCR for the detection of porcine cytokines1. Journal of Immunological Methods 306: 16–27.
Kozlov, A.V., A. Bini, A. Iannone, I. Zini, and A. Tomasi. 1996. Electron paramagnetic resonance characterization of rat neuronal nitric oxide production ex vivo. Methods in Enzymology 268: 229–236.
Fitzal, F., H. Redl, W. Strohmaier, E.R. Werner, and S. Bahrami. 2002. A 4-amino analogue of tetrahydrobiopterin attenuates endotoxin-induced hemodynamic alterations and organ injury in rats. Shock 18: 158–162.
Piel, D.A., C.S. Deutschman, and R.J. Levy. 2008. Exogenous cytochrome c restores myocardial cytochrome oxidase activity into the late phase of sepsis. Shock 29: 612–616.
Levy, R.J. 2007. Mitochondrial dysfunction, bioenergetic impairment, and metabolic down-regulation in sepsis. Shock 28: 24–28.
Miller, I., M. Gemeiner, B. Gesslbauer, A. Kungl, C. Piskernik, S. Haindl, S. Nurnberger, S. Bahrami, H. Redl, and A.V. Kozlov. 2006. Proteome analysis of rat liver mitochondria reveals a possible compensatory response to endotoxic shock. FEBS Letters 580: 1257–1262.
Watanabe, E., J.T. Muenzer, W.G. Hawkins, C.G. Davis, D.J. Dixon, J.E. McDunn, D.J. Brackett, M.R. Lerner, P.E. Swanson, and R.S. Hotchkiss. 2009. Sepsis induces extensive autophagic vacuolization in hepatocytes: a clinical and laboratory-based study. Laboratory Investigation 89: 549–561.
Crouser, E.D., M.W. Julian, J.E. Huff, J. Struck, and C.H. Cook. 2006. Carbamoyl phosphate synthase-1: a marker of mitochondrial damage and depletion in the liver during sepsis. Critical Care Medicine 34: 2439–2446.
Meisner, M., V. Muller, Z. Khakpour, E. Toegel, and H. Redl. 2003. Induction of procalcitonin and proinflammatory cytokines in an anhepatic baboon endotoxin shock model. Shock 19: 187–190.
Brown, L.A., F.L. Harris, and D.M. Guidot. 2001. Chronic ethanol ingestion potentiates TNF-alpha-mediated oxidative stress and apoptosis in rat type II cells. American Journal of Physiology. Lung Cellular and Molecular Physiology 281: L377–L386.
Autelli, R., S. Crepaldi, S.D. De, M. Parola, G. Bonelli, and F.M. Baccino. 2005. Intracellular free iron and acidic pathways mediate TNF-induced death of rat hepatoma cells. Apoptosis 10: 777–786.
Richter, C., V. Gogvadze, R. Laffranchi, R. Schlapbach, M. Schweizer, M. Suter, P. Walter, and M. Yaffee. 1995. Oxidants in mitochondria: from physiology to diseases. Biochimica et Biophysica Acta 1271: 67–74.
Matejovic, M., A. Krouzecky, R. Rokyta Jr., J. Radej, H. Kralova, V. Treska, P. Radermacher, and I. Novak. 2007. Effects of combining inducible nitric oxide synthase inhibitor and radical scavenger during porcine bacteremia. Shock 27: 61–68.
Tosaki, A., and D.K. Das. 2002. The role of heme oxygenase signaling in various disorders. Molecular and Cellular Biochemistry 232: 149–157.
Rothfuss, A., P. Radermacher, and G. Speit. 2001. Involvement of heme oxygenase-1 (HO-1) in the adaptive protection of human lymphocytes after hyperbaric oxygen (HBO) treatment. Carcinogenesis 22: 1979–1985.
Morse, D., and A.M. Choi. 2002. Heme oxygenase-1: the “emerging molecule” has arrived. American Journal of Respiratory Cell and Molecular Biology 27: 8–16.
Brown, G.C., and V. Borutaite. 2002. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radical Biology & Medicine 33: 1440–1450.
Brown, G.C. 1999. Nitric oxide and mitochondrial respiration. Biochimica et Biophysica Acta 1411: 351–369.
Dungel, P., R. Mittermayr, S. Haindl, A. Osipov, C. Wagner, H. Redl, and A.V. Kozlov. 2008. Illumination with blue light reactivates respiratory activity of mitochondria inhibited by nitric oxide, but not by glycerol trinitrate. Archives of Biochemistry and Biophysics 471: 109–115.
Singer, M., V. De Santis, D. Vitale, and W. Jeffcoate. 2004. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet 364: 545–548.
Singer, M., and D. Brealey. 1999. Mitochondrial dysfunction in sepsis. Biochemical Society Symposia 66: 149–166.
Crouser, E.D. 2004. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion 4: 729–741.
Acknowledgement
The authors wish to thank Christina Piskernik, Anna Khadem, Zafar Khakpour, Karl Kropik, Tatjana Morton, Richard Kellner, and Sabine Vagac for their expert technical assistance with the experiments, as well as Sophia Ostermeier, Kim Erwes, and Lisa Landskron, all students at the Veterinary University Vienna, for their skilled technical assistance in determining the heme oxygenase activity in tissue samples. There were no external funding sources supporting this study.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
A. V. K. designed the study and wrote the manuscript; E. C. performed the surgical part of the experiment and contributed to the manuscript; E. G. supervised data analysis and developed the instrument for high-resolution respirometry; I. K. established and performed the ROS assay, analyzed the data, and made figures; H. R. designed the study and contributed to the manuscript; J. C. D. supervised data analysis and contributed to the manuscript; M. v. G. designed the study, supervised the experiment, and contributed to the manuscript; M. J. established and performed the immunoassays, analyzed the data, and made figures; P. R. designed the study and contributed to the manuscript; R. T. H. established and performed the RT-PCR, analyzed the data, and made figures; S. B. wrote the manuscript, designed the study, and supervised the experiment; S. H. established and performed the high-resolution respirometry, analyzed the data, and made figures; T. E. established and performed the EPR-based free iron assay, analyzed the data, and made figures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kozlov, A.V., van Griensven, M., Haindl, S. et al. Peritoneal Inflammation in Pigs is Associated with Early Mitochondrial Dysfunction in Liver and Kidney. Inflammation 33, 295–305 (2010). https://doi.org/10.1007/s10753-010-9185-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-010-9185-4