Abstract
Diverse macrovegetation can provide heterogeneous habitats for benthic diatoms. The removal of macrophytes as direct plant control, however, can be considered as a threat, which can even lead to remarkable microhabitat alterations. Lake Tisza (Hungary) has a high nature conservation value, but it is also an important recreation centre, which is why very delicate water management is necessary including vegetation thinning. Here, we studied the importance of microhabitat heterogeneity (emergent, submerged and floating macrophytes) in maintaining diverse periphytic diatom assemblages. We hypothesized that the substrate type has greater influencing role on the composition and diversity of diatoms than the lake heterogeneity related to basins. We also assumed that floating vegetation hosts the most different and least diverse diatom assemblages. Our results mostly proved these hypotheses. Heterogeneous assemblages were formed on the different substrates (support hypothesis), however, the basin level differences were also detected (reject hypothesis). Our results also highlighted, that macrophyte species with lesser morphological complexity hosted the least diverse periphytic assemblages (support hypothesis). However, many unique and red list taxa were found on floating plants (reject hypothesis). These findings emphasize the key role of microhabitat complexity in maintaining diverse and healthy functioning of microbial assemblages in a multi-purpose reservoir.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Like healthy ecosystems, human society is built on, inter alia, biodiversity (Díaz et al., 2006), however, our knowledge about the variety of life on Earth is not even. While nature conservation studies usually focus on large animals and other organisms, especially on those that are used in human activities, aquatic and subterranean biota, invertebrates or microorganisms including algae, are underrepresented (MEA, 2003). At the same time, freshwaters are strongly threatened by the rapid diversity loss, which can now be considered an “invisible tragedy” below the water surface (Richter et al., 1997; Reid et al., 2019).
Benthic diatoms as important primary producers (Wetzel, 1983; Stevenson, 1996) play a key role in freshwaters, especially in lakes (Cattaneo & Kalff, 1979). They are usually one of the most abundant components of epiphyton (Vermaat, 2005), and may even constitute a significant proportion of the total periphytic biomass (Vadeboncoeur & Steinman, 2002; Sánchez et al., 2017). In addition, even though diatoms are protected by their silica frustules against grazing, they are still an excellent food source for both zooplankton and zoobenthos (Kuiper et al., 2015; Neury-Ormanni et al., 2020) due to their high protein and polyunsaturated fatty acid content (Tenore, 1989; Marella et al., 2020). To perform these functions in ecosystems, well-developed and diverse diatom assemblages are required (B-Béres et al., 2022), the formation of which (besides nutrients and light) can be strongly influenced by the quality and quantity of substrates.
Freshwater macrophytes are one of the most important substrates for periphytic organisms, especially in lakes (Vadeboncoeur & Steinman, 2002) and lowland watercourses (Várbíró et al., 2020). Diverse and complex macrophyte community can drive the periphytic algal community towards increased abundance (Hao et al., 2017), biomass (Gross et al., 2003) and species diversity (Biolo & Rodrigues, 2013), simply because it facilitates settlement, growth and mobility of algae or enables predator avoidance (Tokeshi & Arakaki, 2012). The composition and biomass of benthic algal assemblages, however, are highly controlled by the basic nature of substrate: The difference in the shape and the structural complexity of macrophytes, i.e. presence/number of thorns and the importance of edges, can result in differences in the structure and diversity of benthic algal assemblages (Ferreiro et al., 2013; Osório et al., 2019). Vertical extension of macrophytes, such as emergent, submerged and rooted-floating plants, is also an important driver of algal colonisation, as the amount of light decreases and its quality changes with depth and transparency (Middelboe & Markager, 1997). While submerged macrophytes can provide a variety of habitats for periphyton due to their most complex morphological structure (e.g. compound leaves and floating adventitious roots) (Fernandes et al., 2016), highly variable algal assemblages can be formed on rooted-floating plants (Rojas & Hassan, 2017). These species can also be considered one of the most disturbed microhabitats for algae due to their flushing upper surface. On the other hand, they can be favoured by diatoms which have the ability to survive unstable, aerophilous conditions (Falasco et al., 2016). In addition, algae living on the underwater surface of free-floating leaves have to cope with intense shading. Based on all these, structural complexity of host plants and their life forms seems essential for structuring periphytic algae (Dos Santos et al., 2013).
While the characteristics of species units, i.e. traits have been used in terrestrial vegetation and phytoplankton researches for decades, the trait-based approach has only become widespread over the last 15 years in diatom studies (see more Tapolczai et al., 2016). This approach, inter alia, allows comparing the effect of the same natural or anthropogenic processes (e.g. nutrient load, salinization or climate change) even in different ecoregions. This helps recognizing global forces and processes jeopardize aquatic ecosystems all over the World (Török et al., 2016). In addition, the diverse trait composition of assemblages (high functional diversity) is essential for the proper functioning of ecosystem functions, such as productivity and resistance to invasions (Mason et al., 2005).
Lake Tisza (Kisköre Reservoir) is the second largest standing water in Hungary and in the Carpathian Basin, which has four differently managed basins serving different purposes (Kókai et al., 2019). As part of both national protection (Ministerial Order, 2001) and the UNESCO World Heritage Convention (UNESCO, 1999), the conservation of bird habitats and nesting sites is a priority here. For this reason, this lake is plagued by water quality problems from time to time, due to the eutrophication in the highly protected basin characterized by the highest occurrence of birds. In addition to its high nature conservation value, Lake Tisza also provides other ecosystem services as it is an important recreational centre for anglers, swimmers, canoers, kayakers, etc. (Kókai et al., 2019). In order to make the lake suitable for multi-purpose services, very delicate water management must be implemented including water level control and summer vegetation thinning. Diverse aquatic macrophyte vegetation develops every summer on Lake Tisza, which can achieve such a high density and biomass that hinder many water usage and recreation activities (web 1). Annual vegetation thinning is a common practice in Lake Tisza, but in the recent years, there has been a need for more frequent thinning affecting larger areas. However, the removal of macrophytes in order to directly control plants can be considered as a threat, which can even lead to microhabitat degradation (Thomaz & Cunha, 2010) that ultimately results in diversity loss and reduced functionality. Extended macrovegetation, however, also provides heterogeneous habitats for aquatic assemblages (spiders—Raizer & Amaral, 2001; invertebrates—Taniguchi et al., 2003; birds—Paillisson et al., 2006; fishes—Padial et al., 2009) including periphyton (Fernandes et al., 2016) by creating great variety of physical structures. Due to the above mentioned general dominance of diatoms in the periphyton and their importance as food sources, it is important to know, how vegetation thinning influences on their composition and diversity. Finally, compositional changes in diatom assemblages may affect the structure and diversity at higher trophic levels. Therefore, the role of diatoms in improving practice of conservation seems to be well established.
Here, we aimed to highlight the importance of microhabitat heterogeneity (i.e. different types of macrophytes as emergent, submerged and floating) in maintaining diverse periphytic diatom communities. In our former paper (Kókai et al., 2019), we studied the influence of seasonality (temporal effect) and different management control (spatial effect) of basins on the taxa composition and the diversity of benthic diatoms. We involved the analyses periphytic assemblages formed on only one life form type of macrophytes, i.e. on emergent plant. We revealed a more or less pronounced spatial homogeneity in the taxonomic composition of diatom assemblages in Lake Tisza, furthermore the biodiversity was also slightly influenced by the spatial effect. Based on these results (Kókai et al., 2019) and the above-mentioned role of life forms of macrophytes in shaping composition of benthic algal assemblages, we hypothesized the followings:
(H1)
The type of substrate (microhabitat) has greater influencing role on the composition and diversity of periphytic diatoms than the spatial effect related to the different management of basins,
(H2)
Floating macrophytes show the most pronounced compositional alterations at both taxonomic and trait levels,
(H3)
Floating vegetation hosts the least diverse benthic algal assemblages.
Materials and methods
Sampling setup and measurements
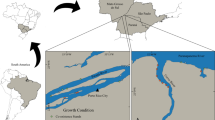
Epiphyitic diatom samples were collected between 2016 and 2019, twice a year (in June and in August), in the four basins of the lake (Fig. 1): Tiszavalk basin (47°40′14.7″N, 20°42′56.4″E)—highly protected, Poroszló- (47°36′39.2″N, 20°40′27.8″E) and Sarud basins (47°35′00.7″N, 20°39′11.5″E) – moderately protected, Abádszalók basin (47°29′53.1″N, 20°35′44.3″E)—slightly protected (Kókai et al., 2019). Beside different basin management, spatial heterogeneity of the reservoir can be observable in the basins (Kókai et al., 2019). While the open water surface is the highest in the Abádszalók and Sarud basins (60–65%), it is the smallest in the Tiszavalk and Poroszló basins (30–35%). In the four basins, the main emergent taxa are Phragmites australis (Cav.) Trin. ex Steud. and Typha spp., while the dominant submerged taxa are Potamogeton spp. and Ceratophyllum spp. As rooted-floating species, Trapa natans L. is the most abundant and characteristic in Lake Tisza. During the sampling period no significant difference was found in the inorganic nitrogen, total phosphorus and chloride ion concentration, as well as water temperature, pH and oxygen saturation between the basins. Other parameters, however, were significantly higher in the Tiszavalk basin (orthophosphate and total nitrogen content) or in the Abádszalók basin (transparency) (Supplementary Table 1). The physical and chemical environment that strongly and directly surrounds the different life forms of macrophytes (emergent, submerged and rooted-floating) was not measured in this study.
Substrates were characteristic aquatic plants, belonging to three life-forms, emergent (Phragmites australis, Typha angustifolia L.), submerged (Potamogeton perfoliatus L.) and floating-leaved/rooted-floating (Trapa natans) macrophytes. In our study, macrophytes as substrates have been selected based on their life form and dominance within the lake, however, these plants also have different structural complexity. The most “complex” (with many edges) is the floating-leaved species, while the less is the emergent ones (mostly a “tube”) (in part according to Ferreiro et al., 2013). All three types of macrophytes were sampled from the same spot in the given basin. During one sampling, i.e. on the same day, we collected altogether 12 samples (4 basins × 3 types), the only exception was the early summer period in 2017 when submerged substrates were not available in the Sarud and Abádszalók basins. This means that the total number of samples was 94: 2 per year per site × 4 year × 3 life form types of macrophytes × 4 basins (except in June in 2017). Sampling and preservation were performed according to the European standard (EN 13946). Samples from the emergent macrophytes were collected from the underwater stem of the plant (5–5 stems per sampling events): the biofilm of a 15–20 cm stem section was removed with a toothbrush and then washed into a sampling bottle filled with tap water. In the case of Potamogeton perfoliatus, 10–10 shoots (stem and leaves) per sampling sites were collected (no rhizome and roots), while 10–10 floating leaves per rosette of Trapa natans (5–5 rosette per sites) were used as substrate during sampling. The plants (leaves, shoots) were placed in a plastic sampling box filled with sterilized tap water, then shaken to remove the weakly attached algae. After that, strongly attached algae were removed with a toothbrush. Periphytic samples were preserved in the field with acetate-free Lugol’s solution, then checked in the laboratory to see if they contained a sufficient amount of living cells, i.e. the ratio of frustules with organic matter was over 90–95%.
Preparation of the samples was conducted by hot hydrogen-peroxide method using Naphrax for embedding (EN 13946). At least 400 diatom valves were identified in each sample (EN 14407) at 1000–1600-fold magnification (Leica DMRB microscope) according to the following references: Krammer & Lange-Bertalot (1997a,b, 2004a,b), Potapova & Hamilton (2007), Bey & Ector (2013), Stenger-Kovács & Lengyel (2015).
Data processing and analyses
Diatom taxa were classified into five traits (Supplementary Table 2): length–width (L/W) ratio (6 categories; Stenger-Kovács et al., 2018), cell size (5 categories; Berthon et al., 2011), attachment (3 categories; Lange et al., 2016), life-form and guild (3 and 4 categories, respectively; Rimet & Bouchez, 2012). Endangered taxa were listed into threatened categories according to the German diatom-specific Red List (3 categories; Hofmann et al., 2018).
To compare the taxonomic and trait composition of the three microhabitats, we performed non-metric multidimensional scaling (NMDS; Canoco 5.0; ter Braak and Šmilauer, 2002). For the trait-based analyses, the community-weighted mean (CWM) matrix was used, in which the mean trait values in the community were weighted by the relative abundances of the species. Permutational multivariate analysis of variance (PERMANOVA) was performed on the community matrix after NMDS analyses to test the statistically significant differences in terms of composition (Anderson, 2001). The percentage breakdown of average dissimilarity between the groups was determined by similarity percentage (SIMPER) analysis using the Bray–Curtis dissimilarity index (Clarke, 1993). The PERMANOVA and SIMPER analyses were performed using Past 4.09 (Hammer et al., 2001).
Species richness (Taxa_S), Shannon diversity (H) and species evenness (JEvenness) were calculated to compare the taxonomic diversities of assemblages (Pileou, 1975). These metrics were calculated using Past software (version 4.09; Hammer et al., 2001). Functional diversity metrics as functional richness (FRich), functional evenness (FEve) and functional divergence (FDiv) were estimated as described by Villéger et al. (2008), while functional dispersion (FDis) was calculated according to Laliberté and Legendre (2010). R environment was used for the calculation of these functional diversity metrics (Laliberté & Legendre, 2010; version 3.5.2; R Core Team, 2019).
In the case of normal distribution, one-way ANOVA and in case of non-normal distribution, Kruskal–Wallis were used to compare the diversity characteristics between aquatic plant life forms and between differently managed basins (ter Braak & Šmilauer, 2002; using Past 4.09). The fixed factors were life forms and basins, while the dependent variables were the taxonomic and functional diversity metrics as Taxa_S, Shannon diversity (H), species evenness (JEvenness), functional richness (FRich), functional evenness (FEve), functional divergence (FDiv) and functional dispersion (FDis).
Results
Taxonomic composition
In the 94 samples, a total of 288 taxa were identified: 286 to species and 2 to genus level; 150 species were found on all three substrates. 19 of them were proved to be characteristic elements of the total periphyton community (occurred at least half of the samples/substrates with abundance > 5%; Supplementary Table 3). Altogether 231 diatom taxa were found on the emergent (32 samples), 197 on the submerged (30 samples) and 210 on the floating-leaved substrates (32 samples). Between substrates, number of common species was 165 (emergent-submerged), 163 (emergent-floating) and 172 (submerged-floating), respectively. In contrast, 87 species occurred only on one type of plants: there were 53 unique species present on emergent, 9 on submerged and 25 on floating macrophyte substrates. Approximately 90% of these species were present in only 1 or 2 samples of the given microhabitat, and their abundance was typically below 1%.
A total of 60 red list species were found in the samples (Supplementary Table 2), of which 31 taxa occurred only on one type of plants: 21 on emergent, 2 on submerged and 7 on floating substrates. While the highest taxa number (43) and cumulative mean abundance (13.07%) were also found on the emergent macrophytes, in the other microhabitats, frequency of endangered taxa was very similar (taxa number 29 and 34, abundance 9.94% and 9.79%, respectively).
Regarding the taxa composition of basins, 181 taxa occurred in Tiszavalk-, 188 in Poroszló-, 216 in Sarud- and 192 in Abádszalók basin. The highest average taxa number was found in the submerged samples in both sampling periods in the case of Tiszavalk-, Poroszló- and Abádszalók basins. The number of common species was 136–155 between basins (Supplementary Table 2), and 80 taxa were found in only one basin (15 in Tiszavalk basin, 12 in Poroszló basin, 33 in Sarud basin and 20 in Abádszalók basin, respectively). Most of these species were rare (1–2 samples) and less abundant (< 1%).
Although there was almost a complete overlap between microhabitats according to the taxonomy-based NMDS analyses (Fig. 2a), the PERMANOVA analyses revealed significant differences between them (p = 0.0277). The pairwise analyses, however, did not reveal compositional differences between the assemblages formed on submerged and floating macrophytes (Supplementary Table 4). In the case of basins, there was a more visible separation (Fig. 2b), which was also supported by the PERMANOVA (p = 0.0001) (Supplementary Table 5). The SIMPER analyses revealed that species as Achnanthidium minutissimum (Kützing) Czarnecki, Aulacoseira distans (Ehrenberg) Simonsen, Diadesmis confervacea Kützing, Gomphonema parvulum (Kützing) Kützing and Melosira varians C.Agardh contributed the most (contribution% > 4) to the compositional differences among substrates (Supplementary Table 4). In addition to these species, two other taxa (Cocconeis lineata Ehrenberg and Gomphonema pumilum (Grunow) E.Reichardt & Lange-Bertalot) also highly contributed to differences among basins (contribution% = 4.05 and 2.898, respectively; Supplementary Table 5).
Taxonomic composition of microhabitats (a) and basins (b) according to NMDS. OMNIDIA codes represent dominant species (abundance ≥ 5%). Green circle–emergent, blue square–submerged, orange diamond–floating macrophytes (a); orange circle–Tiszavalk basin, blue square–Poroszló basin, green diamond–Sarud basin, red star–Abádszalók basin (b)
Trait composition
All trait categories were found both in all types of substrates and in all basins, only their proportions were different. Similar to taxa composition, there was also an overlap in trait composition between substrates (Fig. 3a) and the PERMANOVA revealed marginally significant alterations between substrates (p = 0.0838; Supplementary Table 6). Based on pairwise analyses, there were no significant differences in the composition between assemblages formed on floating and submerged, or floating and emergent macrophytes (Supplementary Table 6). The samples, however, collected from submerged macrophytes can be characterized by the strongly elongated (LW6), and/or colonial, high profile species. In contrast, the smallest (S1), and/or unicellular, and/or strongly attached, and/or low profile taxa and/or moderately attached species were rather related to the emergent plants (Fig. 3a).
Functional composition of microhabitats (a) and basins (b) according to NMDS. Green circle–emergent, blue square–submerged, orange diamond–floating macrophytes (a); orange circle–Tiszavalk basin, blue square–Poroszló basin, green diamond–Sarud basin, red star–Abádszalók basin (b). Length–width (L/W) ratio—LW1, LW2, LW3, LW4, LW5, LW6; cell size—S1, S2, S3, S4, S5; strength of attachment—low attachment, medium attachment, high attachment; life-form—unicellular, filamentous, colonial; guild—low profile, high profile, motile, planktic
The basins were separated from each other, especially the Sarud- and Abádszalók basins (Fig. 3b). The PERMANOVA analysis showed significant differences among them (p = 0.0001; Supplementary Table 7). However, assemblage composition did not reveal pairwise differences between the Poroszló- and Sarud basins (Supplementary Table 7). Colonial or filamentous high profile, and/or LW6, and/or planktic, and/or weakly attached taxa characterized the Sarud basin, while LW3 and/or, strongly attached, and/or unicellular, and/or small sized (S1) and/or low profile traits were indicative to the Abádszalók basin (Fig. 3b).
Accordingly, the most abundant taxa of the mentioned trait categories were the followings: LW6–Eunotia bilunaris (Ehrenberg) Schaarschmidt (max. abundance 5.47%), colonial–Diadesmis confervacea (max. abundance 41.42%), high profile–Melosira varians (max. abundance 68.31%), medium attachment–Gomphonema pumilum (max. abundance 46.19%), unicellular–Cocconeis placentula Ehrenberg spp. (max. abundance 70.48%), S1–Achnanthidium minutissimum (max. abundance 64.2%), motile–Epithemia adnata (Kützing) Brébisson (max. abundance 24.7%).
Diversity
While basin level differences had a significant effect only on taxa number, microhabitats significantly influenced trait diversity but not taxonomic diversity (Table 1). The species richness was the highest in the Poroszló basin, however, neither Shannon diversity nor evenness did differ significantly among the basins or the substrates. Regarding the trait diversity, substrate had significant effect on three of the four components of functional diversity, i.e. functional richness (p = 0.043), functional divergence (p = 0.023) and functional dispersion (p = 0.005; Table 1). While functional divergence (FDiv) reached the highest value on the emergent macrophytes, functional richness (FRich) and functional dispersion (FDis) of submerged microhabitats were the highest compared to the other substrates (Table 1).
Discussion
Compositional changes
The role of aquatic vegetation in habitat structuring for periphyton communities has been demonstrated several times (e.g. de Souza et al., 2015; Fernandes et al., 2016; Leão et al., 2021). Variation in periphytic algal communities, especially in diatom assemblages, implies in changes in large-scale structure of systems that influence dynamics across trophic levels (Hinojosa-Garro et al., 2010). However, opinions differ on how the various life forms of aquatic macrophytes contribute to the formation of diatom assemblages.
Regarding the basically different life form and morphological structure of the sampled host macrophytes in Lake Tisza, the effects of these characteristic plants on the periphytic diatoms’ community could be assessed at microhabitat scale. Here, a more pronounced substrate- than basin-based assemblages heterogeneity was expected (H1). Our results only partially confirmed this hypothesis: More or less heterogeneous assemblages were formed on the different substrates, however, in this study strong basin level differences were detected. Disturbance tolerant taxa, such as Achnanthidium minutissimum (Ács et al., 2006) and other low profile, strongly attached species were definitely characteristics in Abádszalók basin, which is intensively used for recreational activities as water skiing, pleasure boating, swimming (Kókai et al., 2019). In accordance with the findings of Messyasz et al. (2009), that different types of macrophytes maintain microalgal assemblages with different taxonomic compositions, we also found significant structural alterations among the assemblages of various substrates. In addition, trait-based differences in composition were also detected here. These results supported the first part of our second hypothesis (H2): specific morphological features of macrophyte life forms may enhance the formation of different periphyton communities. Furthermore, most unique species were found on emergent plants, thus they seem to provide important niches for more diatom species preferring stable substrate and tolerating variable light intensity including high number of red list taxa than other macrophytic life forms.
However, it should be emphasised that our results partially rejected the second part of H2, because taxonomic and trait composition of benthic diatom assemblages did not show differences between the floating-leaved and the other two macrophyte types and the number of endangered species was high on this substrate. Small, shade tolerant species, such as Achnanthidium minutissimum (Johnson et al., 1997; Díaz Villanueva & Modenutti, 2004), may dominate the underwater surface of free-floating leaves similar to the lower layer of matured biofilm of emergent aquatic plants. Furthermore, the high profile, filamentous or long stalked species, which were characteristic on submerged plants, prefer low disturbance (Passy, 2007), can also find suitable living conditions on the underwater surface of the floating leaves, and since they emerge from the substrate, they can also get enough light there.
Diatom taxa attached to the emergent plants have to cope with various environmental effects both individually and at assemblages level, e.g. changes in light intensity with vertical extension. In line with previous findings (Liess et al., 2009; Stenger-Kovács et al., 2013; Tapolczai et al., 2016) in our study, shade-tolerant low profile species mainly small-sized (S1) and pioneer Achnanthidium minutissimum and Amphora pediculus (Kützing) Grunow, moreover, the larger-sized Cocconeis lineata dominated the biofilm of emergent macrophytes. These strongly attached species can also tolerate grazing by macroinvertebrates (Rimet et al., 2015) which has been suggested as a common biotic pressure in Lake Tisza (Kókai et al., 2019).
High profile colonial (Diadesmis confervacea) and filamentous (Melosira varians), as well as planktic filamentous (Aulacoseira distans) species clearly related to the submerged macrophytes, which cover large areas, creating lentic habitats that are less affected by disturbances caused by abrupt changes in current velocities (Špoljar et al., 2017). These explain why D. confervacea preferring high levels of nutrient supply and reduced flow conditions (van Dam et al., 1994; Kelly & Whitton, 1995; Stenger-Kovács et al., 2013) reached extremely high abundance here. The submerged macrophytes also provided favourable environment for the subsidence of Aulacoseira distans.
Epiphyte grazers (e.g. snails) can be predominant in Trapa natans that can strongly transform communities attached to the floating-leaved macrophytes (Cattaneo et al., 1998). Moreover, in microhabitats formed by floating-leaved macrophytes, there can be an interaction between macrophytes and planktic algae (Scheffer, 1998) resulting in decreased TSS and reduced intensity of photosynthesis (Kókai et al., 2019). Here, weakly attached (Halamphora veneta (Kützing) Levkov) and motile (Nitzschia amphibia Grunow, Nitzschia dissipata (Kützing) Rabenhorst) species achieved the highest frequency and abundance. As subaerophilic species (van Dam et al., 1994—updated in 2011), these taxa also well tolerate water cessation, which occasionally affects floating macrophytes.
Diversity changes
Assembly of periphyton can be driven by physical structure and roughness of host plants (Thomaz et al., 2008; Sultana et al., 2010; Thomaz & Cunha, 2010) resulting in higher species richness on rough substrates (Schneck et al., 2011). On the contrary, in our study, the taxonomic diversity was not influenced by the life form and structure of macrophytes, but, the species richness was controlled by basin level differences in water management, which resulted in the highest diversity in the Poroszló and Sarud basins. As these two basins are characterized by moderate disturbance (i.e. moderately used for recreational activities: Kókai et al., 2019), these findings are in line with Connell's intermediate disturbance hypothesis (1978), which predicts the peak of species diversity at intermediate intensities of disturbance.
Beside taxonomic diversity, diverse functional composition also plays pivotal role in maintenance of ecological processes and healthy functioning of ecosystems (Tilman, 2001; Petchey & Gaston, 2006). In our study, trait distribution of periphytic diatoms was clearly driven by plant characteristics, and as a result, three elements of functional diversity changed significantly on the substrates. While functional divergence reached the highest values on the emergent substrate, functional richness and functional dispersion values were the most elevated on the submerged plants implying that emergent and submerged macrophytes contribute to the functional diversity in different ways, but with equal importance.
Not surprisingly, macrophyte species with greater morphological complexity provide high niche availability for species (Pacini et al., 2009), resulting in more abundant and diverse periphytic communities (Lucena-Moya & Duggan, 2011). In our study, however, the lowest values of the two elements of functional diversity, i.e. functional divergence and functional dispersion, were associated with floating-leaved macrophytes. Although the leaves of T. natans have the most “complex” structure in this study (in part according to Ferreiro et al., 2013), the floating rosette provides also the most disturbed habitat for diatoms. Thus, these results well supported our assumption that the least diverse assemblages populate the floating vegetation (H3). Low functional divergence indicates strong resource competition resulting in inefficient resource use (Mason et al., 2005) in diatom assemblages living on floating macrophytes. In addition, floating substrates created highly disturbed environments for diatoms resulting in significant reduction in the number of specimen within the same trait combinations (Laliberté & Legendre, 2010). Although functional richness was not the lowest here, it was lower than in assemblages living on submerged plants, which highlights the reduction of functional roles within the biofilm (Laliberté & Legendre, 2010).
These results, i.e. differences in biodiversity at taxonomic and functional level, suggest that the distribution of species were strongly influenced by the difference in water management of the basins and the regular artificial water level control. This latest means that the water level of Lake Tisza is regularly drained off in late autumn and is filled up in early spring, which may have a key role in the spread of diatoms and the difference in taxonomic diversity. In contrast, trait-level changes within the assemblages were affected by the microhabitats, i.e. life forms, which strongly supports the importance of special characteristics in the colonization and occupation of different habitats.
Diatom–based perspective in conservation biology
Lake Tisza was primarily created for water storage and flood control. Besides its main function, this standing water/wetland complex represents high nature conservation value acknowledged by both UNESCO and national law. To fulfil all these tasks, water management have to face great challenges, since in addition to complying with water protection regulations, it must also ensure the preservation of nature conservation values, e.g. diverse aquatic habitats for macro-and microorganisms. The absence of significant differences in taxonomic diversity metrics and the relatively high number of unique species, especially on emergent and floating plants, highlighted that all the studied macrophytic life forms contribute to the maintenance of diverse benthic diatom assemblages in Lake Tisza.
In artificial reservoirs, like Lake Tisza, anthropogenic impacts are strongly indicated by diatoms (Falasco et al., 2012), thus, diversity loss and negative changes in assemblage composition stress the need for effective conservation. In accordance, Dunck et al. (2016) found more diverse periphytic algal communities in preserved habitats emphasizing the importance of these researches in conservation initiatives to avoid environmental degradation and save healthy trophic structure. By contrast, our results did not reveal any positive relationship between diversity of periphytic diatom assemblages and protection level of basins. The reason for this is primarily due to that increased abundance of water birds in the most protected area leads to an elevated natural nutrient load driving community structure towards dominance of species indicating higher trophic level and reduced diversity (Kókai et al., 2019). Heino et al. (2009) also found lower diatom diversity in preserved than in managed lotic habitats. These results support the view that a conflict may arise during the parallel implementation of WFD and Habitat Directive on a given area. Since present approaches in nature conservation that mainly focus on preserving habitats of macroscopic organisms do not seem to consider and provide appropriate circumstances for diverse microscopic wildlife. B-Béres et al. (2021) also highlighted that the current protection strategies for maintaining the diversity of benthic diatom assemblages of the Hungarian lentic ecosystems are ineffective. We believe that microhabitat studies like this can help to better understand the needs of microscopic groups. Incorporating this knowledge into nature conservation is the ultimate key to preserving the health of aquatic ecosystems.
With the exception of species richness, no water usage dependent diversity loss was detected in our study, which, however, was the lowest in the most protected basin. In contrast, we demonstrated the key role of microhabitats in structuring composition and functional diversity of diatoms. These results lead us to emphasize that aquatic plant stocks should be carefully managed (i.e. removal, harvesting), and special attention should be paid to associated organisms, as benthic algae. In addition, similarly to highly disturbed streams (Schneck & Melo, 2012), habitat heterogeneity also seems to be particularly important in the maintenance of the diverse periphytic assemblages of Lake Tisza, where the annual water level modification and wide range of recreation activities also create a moderately disturbed environment. Diverse microhabitats, however, result in an increase in functional diversity and can be sources of poorly known and/or possibly endangered diatom species in Lake Tisza.
Conclusion
Our results highlighted that habitat complexity provided by different life forms of macrophytes can be important driver in shaping taxonomic and functional structure of benthic diatom communities in Lake Tisza. These findings also emphasize the key role of microhabitat complexity in maintaining diverse and healthy functioning of microbial assemblages in a multi-purpose standing water. Being aware that it is hard to draw general conclusions based on a regional study, these results help to take a step towards a more holistic approach in nature conservation and water quality management, to involve both macro- and microscopic biological elements and metrics into the planning and decision making processes.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ács, É., K. Szabó, Á. K. Kiss, B. Tóth, G. Záray & K. T. Kiss, 2006. Investigation of epilithic algae on the River Danube from Germany to Hungary and the effect of a very dry year on the algae of the River Danube. Archive Für Hydrobiologie 158: 389–417.
Anderson, M. J., 2001. Permutation tests for univariate or multivariate analysis of variance and regression. Canadian Journal of Fisheries and Aquatic Sciences 58: 3.
B-Béres, V., C. Stenger-Kovács, P. Török & E. T-Krasznai, 2021. Are recent protection strategies sufficient for maintaining diverse freshwater benthic diatom assemblages? Ecological Indicators 127: 107782.
B-Béres, V., Cs. Stenger-Kovács, K. Buczkó, J. Padisák, G. B. Selmeczy, E. Lengyel & K. Tapolczai, 2022. Ecosystem services provided by freshwater and marine diatoms. Hydrobiologia. https://doi.org/10.1007/s10750-022-04984-9.
Berthon, V., A. Bouchez & F. Rimet, 2011. Using diatom life-forms and ecological guilds to assess organic pollution and trophic level in rivers: a case study of rivers in south-eastern France. Hydrobiologia 673: 259–271.
Bey, M. Y. & L. Ector, 2013. Atlas des diatomées des cours d’eau de la région Rhône-Alpes. pp. 1182.
Biolo, S. & L. Rodrigues, 2013. Comparison of the structure of the periphytic community in distinct substrates from a neotropical floodplain. International Research Journal of Plant Science 4: 64–75.
Cattaneo, A. & J. Kalff, 1979. Primary production of algae growing on natural and artificial aquatic plants: a study of interactions between epiphytes and their substrate. Limnology and Oceanography 24(6): 1031–1037.
Cattaneo, A., G. Galanti, S. Gentinetta & S. Romo, 1998. Epiphytic algae and macroinvertebrates on submerged and floating-leaved macrophytes in an Italian lake. Freshwater Biology 39(4): 725–740.
Clarke, K. R., 1993. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18: 117–143.
Connell, J. H., 1978. Diversity in Tropical Rain Forests and Coral Reefs: High diversity of trees and corals is maintained only in a nonequilibrium state. Science 199: 4335.
de Souza, M. L., B. G. Pellegrini & C. Ferragut, 2015. Periphytic algal community structure in relation to seasonal variation and macrophyte richness in a shallow tropical reservoir. Hydrobiologia 755: 183–196.
Díaz, S., J. Fargione, F. S. Chapin III. & D. Tilman, 2006. Biodiversity loss threatens human well-being. PloS Biology 4: e277.
Díaz Villanueva, V. & B. Modenutti, 2004. Experimental analysis of grazing by the mayfly Meridialaris chiloeensis on different successional stages of stream periphyton. International Review of Hydrobiology 89: 263–277.
Dos Santos, T. R., C. Ferragut & C. E. de Mattos Bicudo, 2013. Does macrophyte architecture influence periphyton? Relationships among Utricularia foliosa, periphyton assemblage structure and its nutrient (C, N, P) status. Hydrobiologia 714: 71–83.
Dunck, B., V. M. Algarte, M. V. Cianciaruso & L. Rodrigues, 2016. Functional diversity and trait–environment relationships of periphytic algae in subtropical floodplain lakes. Ecological Indicators 67: 257–266.
EN 13946, 2003. Water quality. Guidance standard for the routine sampling and pretreatment of benthic diatoms from rivers.
EN 14407, 2004. Water quality. Guidance standard for the identification, enumeration and interpretation of benthic diatom samples from running waters.
Falasco, E., L. Ector, E. Ciaccio, L. Hoffmann & F. Bona, 2012. Alpine freshwater ecosystems in a protected area: a source of diatom diversity. Hydrobiologia 695: 233–251.
Falasco, E., E. Piano & F. Bona, 2016. Diatom flora in Mediterranean streams: flow intermittency threatens endangered species. Biodiversity and Conservation 25: 2965–2986.
Fernandes, U. L., E. C. C. Oliveira & S. R. Lacerda, 2016. Role of macrophyte life forms in driving periphytic microalgal assemblages in a Brazilian reservoir. Journal of Limnology 75(1): 44–51.
Ferreiro, N., A. Giorgi & C. Feijoó, 2013. Effects of macrophyte architecture and leaf shape complexity on structural parameters of the epiphytic algal community in a Pampean stream. Aquatic Ecology 47: 389–401.
Gross, M., C. Felbaum & A. Graf, 2003. Epiphyte biomass and elemental composition on submersed macrophytes in shallow eutrophic lakes. Hydrobiologia 506: 559–565.
Hammer, Ø., D. A. T. Harper & P. D. Ryan, 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: 9.
Hao, B., H. Wu, Y. Cao, W. Xing, E. Jeppesen & W. Li, 2017. Comparison of periphyton communities on natural and artificial macrophytes with contrasting morphological structures. Freshwater Biology 62: 1783–1793.
Heino, J., J. Ilmonen, J. Kotanen, H. Mykrä, L. Paasivirta, J. Soininen & R. Virtanen, 2009. Surveying biodiversity in protected and managed areas: algae, macrophytes and macroinvertebrates in boreal forest streams. Ecological Indicators 9: 1179–1187.
Hinojosa-Garro, D., C. F. Mason & G. J. C. Underwood, 2010. Influence of macrophyte spatial architecture on periphyton and macroinvertebrate community structure in shallow water bodies under contrasting land. Fundamental and Applied Limnology 177: 19–37.
Hofmann, G., H. Lange-Bertalot, M. Werum & R. Klee, 2018. Rote Liste und Gesamtartenliste der limnischen Kieselalgen (Bacillariophyta) Deutschlands. Rote Liste Der Gefährdeten Tiere, Pflanzen Und Pilze Deutschlands. 70(7): 601–708.
Johnson, R. E., N. C. Tuchman & C. G. Peterson, 1997. Changes in the vertical microdistribution of diatoms within a developing periphyton mat. Journal of the North American Benthological Society 16: 503–519.
Kelly, M. G. & B. A. Whitton, 1995. The Trophic Diatom Index: a new index for monitoring eutrophication in rivers. Journal of Applied Phycology 7: 433–444.
Kókai, Z., G. Borics, I. Bácsi, Á. Lukács, B. Tóthmérész, E. Csépes, P. Török & V. B-Béres, 2019. Water usage and seasonality as primary drivers of benthic diatom assemblages in a lowland reservoir. Ecological Indicators 106: 105443.
Krammer, K. & H. Lange-Bertalot, 1997a. Bacillariophyceae 1, Naviculaceae. In Gerloff, H., J. H. Heynig & D. Mollenhauer (eds), Süsswasserflora Von Mitteleuropa Elsevier, Heidelberg: 876.
Krammer, K. & H. Lange-Bertalot, 1997b. Bacillariophyceae 2, Bacillariaceae, Epithemiaceae, Surirellaceae. In Gerloff, H., J. H. Heynig & D. Mollenhauer (eds), Süsswasserflora Von Mitteleuropa Elsevier, Heidelberg: 596.
Krammer, K. & H. Lange-Bertalot, 2004a. Bacillariophyceae 3, Centrales, Fragilariaceae, Eunotiaceae. In Gerloff, H., J. H. Heynig & D. Mollenhauer (eds), Süsswasserflora Von Mitteleuropa Spektrum Akademischer Verlag, Heidelberg: 576.
Krammer, K. & H. Lange-Bertalot, 2004b. Bacillariophyceae 4, Achnanthaceae. kritische erganzungen zu Achnanthes s.l., Navicula str., Gomphonema, gesamt literaturverzeichnis teil 1–4. In Gerloff, H., J. H. Heynig & D. Mollenhauer (eds), Süsswasserflora Von Mitteleuropa Spektrum Akademischer Verlag, Heidelberg: 437.
Kuiper, J. J., C. van Altena, P. C. de Ruiter, L. P. A. van Gerven, J. H. Janse & W. M. Mooij, 2015. Food-web stability signals critical transitions in temperate shallow lakes. Nature Communications 6: 7727.
Laliberté, E. & P. Legendre, 2010. A distance based framework for measuring functional diversity from multiple traits. Ecology 91(1): 299–305.
Lange, K., C. R. Townsend & C. D. Matthaei, 2016. A trait based framework for stream algal communities. Ecology and Evolution 6: 23–36.
Leão, H., L. C. R. Esdar & B. Dunck, 2021. The role of macrophyte architecture in driving periphytic algal communities in a lowland river in the Brazilian Amazon. Brazilian Journal of Botany 44: 263–272.
Liess, A., K. Lange, F. Schulz, J. J. Piggott, C. D. Matthaei & C. R. Townsend, 2009. Light, nutrients and grazing interact to determine diatom species richness via changes to productivity, nutrient state and grazer activity. Journal of Ecology 97: 326–336.
Lucena-Moya, P. & I. C. Duggan, 2011. Macrophyte architecture affects the abundance and diversity of littoral microfauna. Aquatic Ecology 45: 279–287.
Marella, T. K., I. Y. Lopez-Pacheco, R. Parra-Saldívar, S. Dixit & A. Tiwari, 2020. Wealth from waste: diatoms as tools for phycoremediation of wastewater and for obtaining value from the biomass. Science of the Total Environment 724: 137960.
Mason, N. W., D. Mouillot, W. G. Lee & J. B. Wilson, 2005. Functional richness, functional evenness and functional divergence: the primary components of functional diversity. Oikos 111: 112–118.
MEA–Millennium Ecosystem Assessment, 2003. Ecosystems and human well-being: a framework for assessment, Island Press, Washington DC.
Messyasz, B., N. Kuczyńska-Kippen & B. Nagengast, 2009. The epiphytic communities of various ecological types of aquatic vegetation of five pastoral ponds. Biologia 64: 88–96.
Middelboe, A. L. & S. Markager, 1997. Depth limits and minimum light requirements of freshwater macrophytes. Freshwater Biology 37: 553–568.
Neury-Ormanni, J., C. Doose, N. Majdi, J. Vedrenne, W. Traunspurger & S. Morin, 2020. Selective grazing behaviour of chironomids on microalgae under pesticide pressure. Science of the Total Environment 730: 138673.
Ministerial Order, 2001. Ministry of Environment and Water: Departmental Order 13/2001 (V.9.) on the Protected Species in Hungary.
Osório, N. C., E. R. Cunha, R. P. Tramonte, R. P. Mormul & L. Rodrigues, 2019. Habitat complexity drives the turnover and nestedness patterns in a periphytic algae community. Limnology 20: 297–307.
Pacini, A., S. Mazzoleni, C. Battisti & C. Ricotta, 2009. More rich means more diverse: Extending the ‘environmental heterogeneity hypothesis’ to taxonomic diversity. Ecological Indicators 9: 1271–1274.
Padial, A. A., S. M. Thomaz & A. A. Agostinho, 2009. Effects of structural heterogeneity provided by the floating macrophyte Eichhornia azurea on the predation efficiency and habitat use of the small Neotropical fish Moenkhausia sanctaefilomenae. Hydrobiologia 624: 161–170.
Paillisson, J. M., S. Reeber, A. Carpentier & L. Marion, 2006. Plant-water regime management in a wetland: consequences for a floating vegetation-nesting bird, whiskered tern Chlidonias hybridus. Biodiversity and Conservation 15: 3469–3480.
Passy, S. I., 2007. Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquatic Botany 86: 171–178.
Petchey, O. L. & K. J. Gaston, 2006. Functional diversity: back to basics and looking forward. Ecology Letters 9(6): 741–758.
Pileou, E. C., 1975. Ecological diversity, Wiley, New York.
Potapova, M. & P. B. Hamilton, 2007. Morphological and ecological variation within the Achnanthidium minutissimum (Bacillariophyceae) species complex. Journal of Phycology 43: 561–575.
R Core Team, 2019. R: A language and environment for statistical computing, in: R Foundation for statistical computing. Vienna, Austria. https://www.r-project.org/.
Raizer, J. & M. E. C. Amaral, 2001. Does the structural complexity of aquatic macrophytes explain the diversity of associated spider assemblages? Journal of Arachnology 29: 227–237.
Reid, A. J., A. K. Carlson, I. F. Creed, E. J. Eliason, P. A. Gell, P. T. J. Johnson, K. A. Kidd, T. J. MacCormack, J. D. Olden, S. J. Ormerod, J. P. Smol, W. W. Taylor, K. Tockner, J. C. Vermaire, D. Dudgeon & S. J. Cooke, 2019. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biological Reviews 94: 849–873.
Richter, B. D., D. P. Braun, M. A. Mendelson & L. L. Master, 1997. Threats to imperiled freshwater fauna. Conservation Biology 11: 1081–1093.
Rimet, F. & A. Bouchez, 2012. Life-forms, cell-sizes and ecological guilds of diatomsin European rivers. Knowledge and Management of Aquatic Ecosystems 406: 01.
Rimet, F., A. Bouchez & B. Montuelle, 2015. Benthic diatoms and phytoplankton to assess nutrients in a large lake: complementarity of their use in Lake Geneva (France–Switzerland). Ecological Indicators 53: 231–239.
Rojas, L. A. & G. S. Hassan, 2017. Distribution of epiphytic diatoms on five macrophytes from a Pampean shallow lake: host-specificity and implications for paleoenvironmental reconstructions. Diatom Research. https://doi.org/10.1080/0269249X.2017.1378128.
Sánchez, M. L., P. Rodríguez, A. M. Torremorell, I. Izaguirre & H. Pizarro, 2017. Phytoplankton and periphyton primary production in clear and turbid shallow lakes: influence of the light environment on the interactions between these communities. Wetlands 37: 67–77.
Scheffer, M., 1998. Ecology of Shallow Lakes, Chapman & Hall, London.
Schneck, F. & A. S. Melo, 2012. Hydrological disturbance overrides the effect of substratum roughness on the resistance and resilience of stream benthic algae. Freshwater Biology 57: 1678–1688.
Schneck, F., A. Schwarzbold & A. S. Melo, 2011. Substrate roughness affects stream benthic algal diversity, assemblage composition, and nestedness. Journal of the North American Benthological Society 30: 1049–1056.
Špoljar, M., C. Zhang, T. Dražina, G. Zhao, J. Lajtner & G. Radonić, 2017. Development of submerged macrophyte and epiphyton in a flow-through system: assessment and modelling predictions in interconnected reservoirs. Ecological Indicators 75: 145–154.
Stenger-Kovács, C. & E. Lengyel, 2015. Taxonomical and distribution guide of diatoms in soda pans of Central Europe. Studia Botanica Hungarica 46: 3–203.
Stenger-Kovács, Cs., E. Lengyel, L. O. Crossetti, V. Üveges & J. Padisák, 2013. Diatom ecological guilds as indicators of temporally changing stressors and disturbances in the small Torna-stream, Hungary. Ecological Indicators 24: 138–147.
Stenger-Kovács, C., K. Körmendi, E. Lengyel, A. Abonyi, É. Hajnal, B. Szabó, K. Buczkó & J. Padisák, 2018. Expanding the trait-based concept of benthic diatoms: development of trait- and species-based indices for conductivity as the master variable of ecological status in continental saline lakes. Ecological Indicators 95: 63–74.
Stevenson, R. J., 1996. An introduction to algae ecology in freshwater benthic habitats. In Stevenson, R. J., M. L. Bothwell & R. L. Lowe (eds), Algal Ecology Academic Press, San Diego: 3–30.
Sultana, M., T. Asaeda, M. E. Azim & T. Fujino, 2010. Morphological responses of a submerged macrophyte to epiphyton. Aquatic Ecology 44: 73–81.
Taniguchi, H., S. Nakano & M. Tokeshi, 2003. Influences of habitat complexity on the diversity and abundance of epiphytic invertebrates on plants. Freshwater Biology 48: 718–728.
Tapolczai, K., A. Bouchez & Cs. Stenger-Kovács, J. Padisák & F. Rimet, 2016. Trait-based ecological classifications for benthic algae: review and perspectives. Hydrobiologia 776: 1–17.
ter Braak, C. J. F. & P. Šmilauer, 2002. CANOCO Reference Manual and CanoDraw ForWindows User’s Guide: Software for Canonical Community Ordination (Version 4 .5). Microcomputer Power, Ithaca, NY (Accessed. 2013). http://www.canoco.com.
Tenore, K. R., 1989. Some ecological perspectives in the study of the nutrition of deposit feeders, Ecology of Marine Deposit Feeders. Springer, New York.
Thomaz, S. M. & E. R. Cunha, 2010. The role of macrophytes in habitat structuring in aquatic ecosystems: methods of measurement, causes and consequences on animal assemblages’ composition and biodiversity. Acta Limnologica Brasiliensia 22(2): 218–236.
Thomaz, S. M., E. D. Dibble, L. R. Evangelista, J. Higuti & L. M. Bini, 2008. Influence of aquatic macrophyte habitat complexity on invertebrate abundance and richness in tropical lagoons. Freshwater Biology 53: 358–367.
Tilman, D., 2001. Functional diversity. In Levin, S. A. (ed), Encyclopedia of Biodiversity Academic Press, San Diego: 109–120.
Tokeshi, M. & S. Arakaki, 2012. Habitat complexity in aquatic systems: fractals and beyond. Hydrobiologia 685: 27–47.
Török, P. & E. T-Krasznai, V. B-Béres, I. Bácsi, G. Borics & B. Tóthmérész, 2016. Functional diversity supports the biomass–diversity humped-back relationship in phytoplankton assemblages. Functional Ecology 30: 1593–1602.
UNESCO, 1999. UNESCO World Heritage Committee Nomination Documentation. Available at: http://whc.unesco.org/uploads/nominations/474rev.pdf
Vadeboncoeur, Y. & A. D. Steinman, 2002. Periphyton Function in Lake Ecosystems. The Scientific World Journal 2: 923031.
Van Dam, H., A. Mertens & J. Sinkeldam, 1994. A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Netherlands Journal of Aquatic Ecology 28: 117–133.
Várbíró, G., G. Borics, M. H. Novais, M. M. Morais, F. Rimet, A. Bouchez, K. Tapolczai, I. Bácsi, P. Usseglio-Polatera & V. B-Béres, 2020. Environmental filtering and limiting similarity as main forces driving diatom community structure in Mediterranean and continental temporary and perennial streams. Science of the Total Environment 741: 140459.
Vermaat, J. E., 2005. Periphyton Dynamics and Influencing Factors. In Azim, M. E., M. C. J. Verdegem, A. A. van Dam & M. C. M. Beveridge (eds), Periphyton: Ecology, Exploitation and Management CABI Digital Library, London: 35–49.
Villéger, S., N. W. H. Mason & D. Mouillot, 2008. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89: 2290–2301.
web 1. https://www.freshwater-ecology.com/kozcelu-hinarnoveny-szabalyozas-a-tisza-tavon-kiskorei-tarozo/
Wetzel, R. G., 1983. Attached algal-substrata interactions: fact or myth, and when and how? In Wetzel, R. G. (ed), Periphyton of Freshwater Ecosystems. Developments in Hydrobiology, Dordrecht.
Acknowledgements
The project was financially supported by the National Research, Development and Innovation Office—NKFIH FK 132 142 (VBB) and NKFIH KKP 144068 (PT, ZsNK, ÁL and VBB), by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences BO-00458-20-8 (VBB) and by the ÚNKP-20-5 (VBB), ÚNKP-21-5 (VBB) and ÚNKP-22-5 (VBB) New National Excellence Program of the Ministry for Innovation and Technology from the Source of the National Research, Development and Innovation Fund. We are thankful to Tamás Bozóki for preparation of the map (Fig. 1).
Funding
Open access funding provided by ELKH Centre for Ecological Research. National Research, Development and Innovation Office, NKFIH FK 132 142, Viktória B-Béres, NKFIH KKP 144068, Péter Török, János Bolyai Research Scholarship of the Hungarian Academy of Sciences, BO-00458-20-8, Viktória B-Béres, the Source of the National Research, Development and Innovation Fund, ÚNKP-20-5, Viktória B-Béres, ÚNKP-21-5, Viktória B-Béres, ÚNKP-22-5, Viktória B Béres.
Author information
Authors and Affiliations
Contributions
ZsNK and VBB: developed the structure and drafted key issues of this paper; ZsNK: wrote the manuscript and proved the data; PT and ÁL: carried out the statistical analyses; VBB, GB and IB: helped in writing of the manuscript; VBB and ECs: raised the topic; ECs, ÁL, ETK, IB and VBB: collected samples. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
No human participants and animals were involved in the research.
Additional information
Handling editor: Sidinei M. Thomaz
Guest editors: Viktória B-Béres, Luigi Naselli-Flores, Judit Padisák & Gábor Borics / Trait-Based Approaches in Micro-Algal Ecology
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nemes-Kókai, Z., Borics, G., Csépes, E. et al. Role of microhabitats in shaping diversity of periphytic diatom assemblages. Hydrobiologia 851, 959–972 (2024). https://doi.org/10.1007/s10750-023-05336-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05336-x