Abstract
The invasive Crassula helmsii is rapidly expanding in Europe. Several ecological effects are described, most of which focus on ecosystem functioning and native vegetation but rarely on fauna. In North-western Europe, C. helmsii often invades the breeding habitat of endangered amphibians, such as Epidalea calamita. The spawning rate, egg survival and the speed of larval growth of this toad species in relation to the presence of C. helmsii were studied. In order to unravel causal mechanisms, effects on natterjack toads were related to the effects of C. helmsii presence/absence on temperature and chemical properties of the water. Spawning and egg survival were significantly lower under C. helmsii dominance compared to bare soil conditions, and negatively affected the population size of E. calamita. However, larval growth rate was significantly higher in C. helmsii dominated treatments, which could be beneficial. Differences in water temperature and chemistry were a possible explanation for these effects. It remains unclear whether the population viability of E. calamita is negatively affected when C. helmsii is present. In many areas, however, this plant species completely overgrows and causes desiccation of waterbodies. Therefore, appropriate management measures will be required to protect this toad against this invader.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive alien species are considered to be a major threat to world’s biodiversity (e.g., Hobbs, 2000; McNeely, 2001; Mooney & Cleland, 2001; Clavero & García-Berthou, 2005; Genovesi, 2005; Butchart et al., 2010; Ruckelshaus, 2020). The processes of competition and niche displacement, among others, can eventually result in the local extinctions of populations of endangered species (Mooney & Cleland, 2001; Genovesi, 2005; Blackburn et al., 2019; Rabitsch et al., 2020). The Australian swamp stonecrop Crassula helmsii (T. Kirk) Cockayne, native to Australia and New Zealand, has a high dispersal ability and is regarded as an invasive species in North-western Europe owing to its ability alter habitats through extremely high annual biomass production (Dawson & Warman, 1987; OEPP/EPPO, 2007; Smith and Buckley, 2020). This perennial amphibious weed is increasingly dominating moorlands, dune slacks, lakes and other freshwater to slightly brackish wetlands, thereby threatening protected ecosystems and endangered species (Dawson & Warman, 1987; Leach & Dawson, 1999; OEPP/EPPO, 2007; Newman, 2013; Brouwer et al., 2017; Prinz et al., 2019; Smith & Buckley, 2020). A variety of habitat alterations after invasions by C. helmsii have been described, such as changes in hydrology and water quality (Newman, 2013), reduction of characteristic species richness in aquatic ecosystems (Dawson & Warman 1987; Leach & Dawson, 1999; Hussner, 2009) and effects on plant species (Pilkington, 2016). Other studies concluded that C. helmsii had impacts on biodiversity similar to fast growing native species such as Phragmites australis (Cav.) Trin. ex Steud. (Dean, 2015), whereas in a field study in England no species loss or germination suppression of native plant species was observed (Langdon, 2004). Several studies have described positive effects of invasive alien species, such as providing shelter for invertebrates, tadpoles or increasing the food supply (e.g. Rodriguez, 2006; Grutters et al., 2015; Reyne et al., 2021; Velle et al., 2022). However, such positive effects of the species have not yet been described for C. helmsii, with the exception of Smith & Buckley (2015) who found that C. helmsii increases the rarity scores of macrophyte assemblages in south-east England. As noticed for other aquatic weeds, C. helmsii may contribute to production and regulating ecosystem services. In various European countries, this species is still sold as an aquarium and pond plant (Dawson & Warman, 1987; OEPP/EPPO, 2007). In Europe, the flowering period lasts from June to the end of October. It has been suggested that these flowers may be a source of nectar for insects (Lockton, 2009). Outside of its native range, however, no pollinators have as yet been observed (Dawson & Warman, 1987; Smith & Buckley, 2020).
In North-western Europe, C. helmsii often invades the breeding habitat of several endangered species, such as amphibians (OEPP/EPPO, 2007). Few studies explicitly examined relationships between C. helmsii and animals. Egg-hatching of the newt Lissotriton vulgaris (L., 1758) was delayed on C. helmsii when compared to their preferred native species Nasturtium officinale R.Br. (Langdon, 2004). Diaz (2012) suggested that aquatic invertebrates and fish species were negatively affected by depletion of CO2 in the water column caused by C. helmsii. In contrast to these findings, several studies also reported no effect of C. helmsii invasions on macroinvertebrate diversity (Ewald, 2014; Smith, 2015; Smith & Buckley, 2015). However, knowledge about the effects of C. helmsii is lacking for many endangered species.
Populations of the natterjack toad Epidalea calamita (Laurenti, 1768) may be threatened by C. helmsii invasions. This endangered amphibian species and its habitats are protected according to the European Habitat Directive Annex IV (Council of the European Communities, 1992). In the Netherlands, their populations have declined by 40% since 1950 (Goverse, 2009; Netherlands Enterprise Agency, 2014), and the species has been added to the Dutch Red List of Endangered Species (Ministry of Agriculture, Nature and Food Quality, 2009). The IUCN 3.1 Red List Assessment of E. calamita also recognized that populations are declining, but classified the species as least concern (Beja et al., 2009).
In this paper, the effects of C. helmsii dominance on the reproduction and larval growth of E. calamita were examined using field surveys and an enclosure experiment. Species interactions are expected as both species occupy similar pioneer habitats, characterized as edges of temporary water bodies with gentle slopes, consisting of sandy soils with open patches in vegetation and low plant litter (Beebee, 1979; Dawson & Warman, 1987; Sinsch, 1998; Creemers et al., 2009; Brouwer et al., 2017; Rayne et al., 2021; Van der Loop et al., 2020). Invasions of C. helmsii could affect the suitability of habitats for the reproduction of E. calamita through overgrowing sparsely vegetated soils that are used for egg deposition by this amphibian. As C. helmsii fills up the water column, competition for space increases, which is known to reduce the survival rate of tadpoles (Brady & Griffiths, 2000). Dominant presence of C. helmsii can also profoundly affect water temperature and chemistry, thus possibly affecting microclimate, water quality and/or food availability for E. calamita tadpoles. Compared to sparsely vegetated littoral zones and inundated banks, these bare soil breeding habitats invaded by C. helmsii are expected to show less strong temperature fluctuations due to shading effects at daytime and insulating effects at night time, possibly affecting hatching of eggs as well as speed of larval growth. C. helmsii is also known to absorb a substantial amount of nutrients from the water layer, possibly reducing the productivity of larvae-preferred algae (Newman, 2013; Van Kleef et al., 2017), whereas the succulent vegetative parts of this plant are expected to be inedible to tadpoles. When less food is provided, tadpoles could exhibit a slower growth rate and lower chance of survival (Golay, 1996; Sinsch, 1998). Tadpoles are at increased risk of not reaching metamorphosis before desiccation of pools and rising of dissolved organic substances result in deterioration of water quality, or C. helmsii’s high biomass production leaves insufficient movement space for swimming organisms, such as tadpoles, probably resulting in problems with resource acquisition (Sinsch, 1998). Malnourished tadpoles remain smaller as juveniles generally resulting in a lower survival rate on land, since smaller juveniles dehydrate more quickly and are slower, increasing the risk of predation (Altwegg & Reyer, 2003; Reques & Tejedo, 1997). The dominant presence of C. helmsii can also result in lowered pH and changes in water chemistry (Newman, 2013; Van Kleef et al., 2017). Such acidification may lead to lethal toxic ammonium and aluminium stress in E. calamita tadpoles (Leuven et al., 1986; Fedorenkova et al., 2012).
In order to study the effects of C. helmsii invasion on the reproductive success of E. calamita, field surveys and an enclosure experiment were performed at a breeding site of this toad. We surveyed the spawning of E. calamita at soils dominated by C. helmsii and at bare soils. An in situ enclosure experiment was executed to determine the effect of C. helmsii on egg-hatching and early larval growth. We hypothesized that C. helmsii invasion affects E. calamita’s breeding success through:
-
(i)
reduced spawning (egg deposition) due to the complete overgrowth of its spawning habitat (i.e. bare soils in littoral zones of shallow and temporary waterbodies);
-
(ii)
reduced larval growth resulting from nutrient uptake by plants from the water thereby reducing growth of algae, which are the preferred larval food source;
-
(iii)
reduced larval growth due to alteration of water chemistry resulting in a toxic environment for tadpoles;
-
(iv)
reduced larval growth due to reflection of sunlight and reduced temperature fluctuation.
Materials and methods
Study site
The field surveys and enclosure experiments were conducted in the nature area ‘De Gijzenrooise Zegge’ in the province of Noord-Brabant, the Netherlands (51° 24′ 24.29″ N, 5° 31′ 36.87″ E). This area is characterized by a brooklet flowing through wet grasslands with fens and natural lakes. Recently, the ground water level of the study site has been artificially increased in order to mitigate effects of desiccation. The area is designated as a nature conservation site since it harbours a population of E. calamita. The study site consists of a seasonally inundated area containing a small, shallow fen-like lake of 0.75 ha, fed by rainwater and characterized by sparse vegetation. The waterbody has been restored, and is managed, to maintain in a low succession phase containing sparsely vegetated and bare soils which are used by E. calamita for reproduction (Fig. 1). Approximately 5 to 6 years ago, C. helmsii invaded this site. Due to absence of native vegetation, the species could form dense swards on a large part of the waterbody and its banks creating a situation with C. helmsii dominance alternating with bare soil. Expansion of the plant to bare soils is ongoing with the expectation that eventually these waterbodies will become totally overgrown by the invasive species.
Experimental setup
Field surveys of vegetation and spawning activity
To determine if C. helmsii cover affected spawning of E. calamita, spawn string release was surveyed immediately after the peak of spawning activity, along a 200 m transect on the west bank of the lake in the ‘De Gijzenrooise Zegge’. Here, spawn strings were observed in previous years (pers. observation M. van de Loo). Every 5 m along the same 200 m transect, the cover of C. helmsii and bare mineral soils were estimated in percentages on the 9th of April 2018, in the upper 3 m of the littoral zone. In addition, all spawn strings of E. calamita were counted along this transect in order to characterize spawning preference in relation to vegetation cover. Within a 2 m radius of each recorded spawn string, the cover (%) of C. helmsii was recorded as well.
Enclosures
On the 9th of April 2018, 40 enclosures were placed in a transect parallel to the shore of the natural lake in the ‘De Gijzenrooise Zegge’ (Table 1). The area in which all enclosures were placed was 0.04 ha and each enclosure was located at a maximum distance of 5 m from other enclosures, in order to control for site effects. The enclosures consisted of a black PVC tub without a bottom. These tubs had a semi-cylindrical shape with a diameter of 53 cm at the widest part, and 48 cm at the narrowest part (in total ca. 56.13 l). All tubs were 48 cm high, of which 20 cm was pressed into the soil to prevent the escape of tadpoles. In total, 20 enclosures were placed at soils with a vegetation that was dominated by C. helmsii (cover: 100%, average water depth 25.5 cm). The other 20 enclosures were placed on sparsely vegetated soils (native vegetation absent, cover of C. helmsii < 2%, average water depth 22 cm; hereafter: bare soil). At the start of the experiment, all potential predators (i.e. Anisoptera and Notonecta sp.) of E. calamita eggs and tadpoles were removed from the enclosures. The top of the enclosures was covered with woven net with a mesh size of 2 × 2 cm to prevent predation of tadpoles by birds. Eggs of E. calamita were incubated in 10 enclosures on bare soil and 10 on C. helmsii dominated soil. The remaining 10 enclosures on bare soil and 10 on C. helmsii dominated soil were not incubated with eggs. This experimental set-up allowed comparisons of water chemistry and temperature in enclosures with and without 100 eggs per enclosure.
Egg hatching
On the 10th of April 2018, circa 2.000 eggs of E. calamita were collected at the study site from the same shallow lake. 100 eggs per enclosure were separated from the sting by hand (wearing latex gloves for protection of the eggs) and incubated in 10 enclosures with C. helmsii dominated soils and 10 enclosures on bare soil. The eggs in each enclosure originated from at least five strings to ensure sufficient genetic diversity of eggs and to compensate for possible differences in development time. After 10 days of incubation in the enclosures, eggs either hatched, resulting in a tadpole, or clearly failed to develop and died. The number of successfully hatched eggs was determined in each enclosure by counting live tadpoles.
Larval growth
Larval growth following hatching was monitored for 43 days until the last tadpoles reached early metamorph stage. From each enclosure containing E. calamita tadpoles, 15 individuals were randomly caught once a week between 20th of April and 7th of June 2018, using a small landing net. Each captured tadpole was then transferred by hand, wearing powderless latex gloves in a water-filled cuvette. Subsequently, total length was measured for each individual by placing the cuvette on plasticized millimetre paper and taking a picture. This ensured an accurate measurement while minimizing disturbance for the tadpoles. However, this method did not allow for individual follow-up measurements of each tadpole. The data collected was a set of measured lengths of the respective enclosure’s tadpole population. After each measurement, tadpoles were returned to their respective enclosure. To prevent metamorphosed toads from drowning in the enclosures, any tadpoles that reached early metamorphosis were released into their natural habitat outside the enclosures immediately after the last measurement.
Water chemistry
To determine effects of enclosures, presence of tadpoles and/or C. helmsii dominance on water chemistry, water samples were taken at the start of the experiment (16th of April 2018) and at three times during the experiment (24th of April, and 9th and 23rd May 2018) in all enclosures (with and without tadpoles present). The pH was measured with a standard combined glass Ag/AgCl pH electrode (Orion Research, Beverly, CA, USA) connected to a pH meter (Tim800; Radiometer analytical, Lyon, France). An auto-analyser 3 system (Bran & Lubbe, Norderstedt, Germany) was used to measure concentrations of nitrate (NO3−), ammonium (NH4+), sodium (Na+), chloride (Cl−) and aluminium (Al3+) colourimetrically using hydrazine sulphate (Kamphake et al., 1967).
Water temperature
Water temperature was measured using HOBO Pendant UA-001-08 data loggers placed in all enclosures containing tadpoles. Water temperature was registered every 15 min from 14th of April at 12:00 AM to 20 of May at 11:00 PM. In order to evaluate the effect of the enclosures on water temperature, four additional data loggers were placed outside the enclosures, two on C. helmsii dominated soil and two on bare soils. For each day and each separate logger, we obtained mean temperature (Tmean), maximum (Tmax) and minimum (Tmin) recorded temperature and derived from these the maximum daily temperature fluctuation (Tfluc = Tmax − Tmin) and maximum daily fluctuation scaled by daily mean temperature (Tfluc/mean = Tfluc/Tmean).
Statistical analysis
All data were tested and visualized using the statistical programme R version 4.0.2 (R core Team 2020), using the packages lme4 (Bates et al., 2015), multcomp (Hothorn et al., 2008), AER (Kleiber & Zeileis, 2008), qpcR (Spiess, 2018), car (Fox & Weisberg, 2019), PMCMRplus package (Pohlert, 2021), Gamm4 (Wood & Scheipl, 2020), lawstat (Gastwirth et al., 2020), Hmisc (Harrell & Dupont, 2020) and mgcv (Wood, 2011). The R code and statistical output is added as supplementary material (SM) appendix I (online appendix to this paper).
Egg spawning
We tested whether mean C. helmsii cover significantly influenced number of egg spawn strings encountered using a General Linear Model (GLM) with negative binomial distribution, since data was discrete, not normally distributed (Shapiro–Wilk: W = 0.529, P-value ≤ 0.001) and showed significant overdispersion in an a priori performed poisson GLM model (dispersion factor 40.06, P < 0.001).
Egg-survival
Normality assumptions were met for the number of hatched eggs in enclosures (Shapiro–Wilk: W = 0.970, p-value = 0.759). Therefore, effect of contrasting habitat structure categories (bare soil versus 100% cover of C. helmsii) on number of hatched eggs were tested using a Linear Model (LM).
Larval growth
Initial fitting of linear or generalized linear models on larval growth over time resulted in significant over- or underfitting of our measured data over the time period. We accounted for this by testing effects of contrasting habitat structure categories on larval growth using a Generalized Additive Model (GAM) approach, with the smoothing term modelling the time effect on larval size and the categorical variable (bare soil vs 100% C. helmsii dominance) as additive predictor variable affecting larval size (for model parameters, see SI Table 3). Apart from C. helmsii dominance vs bare soil treatment, the initial number of tadpoles, counted by successfully hatched eggs, at the start of the experiment was hypothesized to affect larval growth rate by competition between tadpoles. Consequently, the initial number of tadpoles was also incorporated in the analysis as a covariate.
We specified several models varying in degree of complexity, including a null-model, main treatments (C. helmsii dominating vs bare soils; both as additive and interacting factor) and the number of initial tadpoles at the start of the experiment (SI Table 4) and selected the best describing model using the respective model BICs. Since data were not normally distributed, continuous but increasing in variance over time, we performed a Gamma distributed GAM with identity link for each model.
Water chemistry analysis
Effects of C. helmsii dominance on water chemistry (pH, alkalinity and seven different water chemistry components, CO2, HCO3−, NO3−, NH4+, Na+, Cl− and Al3+) were analysed. Assumptions for normality were met for pH (Shapiro–Wilk: W = 0.991, P-value = 0.392), HCO3− (Shapiro–Wilk with log transformation: W = 0.991, P-value = 0.392) and NH4+ (Shapiro–Wilk with log transformation: W = 0.994, P-value = 0.820). An ANOVA of a linear model was used to test for treatment effects. Normality assumptions were not met for: NO3−, (Shapiro–Wilk: W = 0.631, P-value < 0.001), Na+ (Shapiro–Wilk: W = 0.783, P-value ≤ 0.001), Cl− (Shapiro–Wilk: W = 0.781, P-value ≤ 0.001) and Al3+ (Shapiro–Wilk: W = 0.565, P-value < 0.001), and therefore a non-parametric Kruskal–Wallis test was used to test for these treatment effects.
Water temperature analysis
Since assumptions for normality of temperature data were not met, we tested for significance of differences between treatments using a Kruskal–Wallis non parametric test, and compared for statistical differences between treatment groups with the Dwass, Steel, Critchlow and Fligner procedure from the PMCMRplus package (Pohlert, 2021).
Results
Egg spawning
During the field survey a total of 48 spawn strings were found in the littoral zone of the western part of the lake in ‘De Gijzenrooise Zegge’. The mean cover (%) of C. helmsii in areas where E. calamita spawn strings were found was significantly lower than that in areas where spawns were absent (30.45% and 1.60% cover, respectively, Fig. 2, z = 5.866, P < 0.001, SI Table 1).
Cover of Crassula helmsii (%) at locations along a 200 m transect (N = 40) and within a radius of 2 m around spawn strings of Epidalea calamita (N = 48) in a natural lake of ‘De Gijzenrooise Zegge’. Boxplots display the first quartile (bottom line of box), median (middle line of box), third quartile (top line of box) and maximum (highest value). Dots indicate outliers in data
Egg-survival
The mean percentage of hatched eggs was significantly higher (LM: t = − 4.543, P ≤ 0.001 N = 20) in enclosures placed on bare soils (87.7%) in comparison to enclosures placed on C. helmsii-dominated soil (65.9%) (Fig. 3 and SI Table 2).
Average hatching success of Epidalea calamita eggs (%) on bare mineral soils (N = 10) and Crassula helmsii dominated soils (N = 10) in field enclosures. Boxplots display the first quartile (bottom line of box), median (middle line of box), third quartile (top line of box) and maximum (highest value). Dots indicate outliers in data
Larval growth
Eggs started hatching on April 20th, and the first metamorphic toads were observed on May 22nd. When comparing all fitted GAM models, the model incorporating different smoothing curves for C. helmsii dominated and bare soil enclosures and including an additive effect of C. helmsii versus bare soil treatment as predictors of length by development time received most support (SI Tables 3 and 4: Model 1; Wi = 0.93). All other models received little support (SI Table 3 and 4: Model 5 (included initial number of tadpoles as additive effect): rel. ll = 0.09; Wi = 0.07) or very low support (SI Tables 3 and 4: all other models specified: Wi < 0.001). Hence, model 1 was used in estimation of parameter effects (SI Tables 3 and 4).
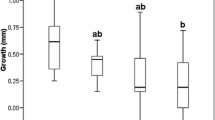
Mean total length of tadpoles was significantly higher in the enclosures with C. helmsii dominated soil than in enclosures with bare soils (SI Table 5; C. helmsii: 14.51; Bare Soil: 13.69; Fig. 4, t = − 13.1, P ≤ 0.001). Average length of tadpoles increased significantly faster under conditions with C. helmsii dominance than with bare soil (see difference between smoothing curves in Fig. 4, calculated from the model parameters of model 1; Tables SI 3,4 and 5).
The total length (mm) of individual Epidalea calamita tadpoles (N = 15; 8 measurements over a period of 28 days) on bare soil enclosures (indicated by brown colour, N = 20) and Crassula helmsii dominated soils (indicated by green colour, N = 20, P ≤ 0.001). Lines indicate the average length of tadpoles. Shading around the lines show the 95% confidence intervals. Day 0 is first day of measuring, April 20th 2018. Measuring data was jittered along y and y axis to increase visibility of individual data points
The development of tadpoles in enclosures dominated by C. helmsii was faster than in enclosures on bare soil (Fig. 4). The onset of metamorphosis of tadpoles in these enclosures started after day 28 and 31, respectively.
Water chemistry
The pH of surface water significantly differed between the four different measuring rounds (F = 17.904, P < 0.001 Table SI_7), this was mainly caused by higher pH values during the last two measurements, and excluding these measurements resulted in no significant pH effect (for test results, see SI Table 9).
Furthermore, the pH was significantly lower in the C. helmsii-dominated enclosures (average 6.30, SE ± 0.02 in water on bare soil and 6.05, SE ± 0.02 in C. helmsii dominated enclosures F = 107.780, P < 0.001, SI Fig. 1, SI Tables 6, 7 and 9).
On the last two measuring dates, ammonium and nitrate concentrations were significantly lower in enclosures containing C. helmsii (respectively: F = 16.120, P < 0.001 and χ2 = 110.600, P ≤ 0.001 SI Figs. 2 and 3, and SI Tables 6, 7, 8 and 10). No effect of presence of E. calamita eggs on this ammonium concentration was found (F = 1.540, P = 0.217). Water temperatures rose above 25 °C and stimulated the decomposition of organic matter and therefore the release of inorganic nitrogenous compounds. Elevated inorganic nitrogen levels were measured in bare soil enclosures on the last two measuring dates. In C. helmsii dominated enclosures this increase was not observed.
During the experiment, evaporation caused a decrease in water levels resulting in significantly increased concentrations of sodium, chloride and aluminium (respectively: χ2 = 122.850, P < 0.001, χ2 = 131.740, P < 0.001 and χ2 = 98.705, SI Tables 6 and 8). The enclosures with C. helmsii dominated soil contained more water in the later stages of the experiment, presumably resulting from lower mean water temperatures (see below) in these enclosures, which reduced evaporation and therefore limiting the concentration effect of evaporation (SI Figs. 4, 5 and 6).
Water temperature
Plots of temporal fluctuations of water temperature and differences between treatments during the experiment are added as supplementary material (online appendix to this paper). Tmean differed between treatments (Kruskal–Wallis rank sum test: χ2 = 9.14, df = 3, P = 0.028), but between-treatment differences were not significant (post-hoc test; P > 0.05 for all comparisons; SI Fig, 7A). Tfluc differed significantly between treatments (Kruskal–Wallis rank sum test: χ2 = 69.44, df = 3, P < 0.001) and was significantly larger on bare soils outside the enclosures compared to bare soil and C. helmsii within the enclosures (SI Fig. 7, panel B; P < 0.001), but not between C. helmsii and bare soil locations outside enclosures (SI Fig. 7, panel B; P > 0.05). Within enclosure treatments, daily temperature fluctuations were significantly smaller in C. helmsii dominated enclosures compared to bare soil (SI Fig. 7, panel B; P < 0.001).
Maximum day-time temperature of water differed significantly between treatments (Kruskal–Wallis rank sum test: χ2 = 23.2, df = 3, P < 0.001), but only between the enclosures and sampling locations outside the enclosures, with higher maxima outside the enclosures (SI Fig. 7). Minimum day time temperature differed significantly between treatments (Kruskal–Wallis rank sum test: χ2 = 16.4, df = 3, P < 0.001), but only was significantly lower in C. helmsii dominated enclosures compared to bare soil enclosures (SI Fig. 7, panel C, P < 0.001).
Mean water temperature scaled daily fluctuation (Tfluc/mean) differed significantly between treatments (Kruskal–Wallis rank sum test: χ2 = 73.8.4, df = 3, P < 0.001) and was significantly higher at sites with bare soil outside enclosures compared to all other treatments (SI Fig. 7 panel D, P < 0.001). Within enclosures, Tfluc/mean of the water was higher in bare soil compared to C. helmsii dominated treatments (SI Fig. 7 panel D, P < 0.001). Crassula-dominated locations outside enclosures showed an intermediate Tfluc/mean, but were not significantly different from bare soil enclosures.
Discussion
The presence of C. helmsii resulted in multiple effects on the reproduction of E. calamita. Egg strings were typically found to be deposited predominantly on locations with a lower cover of C. helmsii. The average percentage of hatched eggs was also found to be lower in enclosures with a dominant soil cover of C. helmsii. Total length of tadpoles was however significantly higher, and growth and development to adult stages was faster in the enclosures with C. helmsii dominated soil.
C. helmsii significantly affects abiotic parameters of the water column where thick and dense vegetation covers the littoral zone (SI Table 6). The pH was significantly lower in C. helmsii dominated enclosures in comparison to bare soil enclosures. At the end of the field measurements, ammonium and nitrate concentrations were significantly lower in enclosures containing C. helmsii. The enclosures with C. helmsii dominated soil retained more water and had lower water temperatures in comparison to the enclosures with bare soil. This resulted in lower evaporation rates and thus lower total evaporation. The higher volume of water loss in enclosures without C. helmsii dominance resulted in a significant increase of ion concentrations (i.e. ammonium, nitrate) at the end of the experiment. Daily temperature fluctuations were indeed significantly smaller in C. helmsii dominated enclosures compared to the bare soil enclosures resulting in a lower minimum day time temperature in C. helmsii dominated enclosures.
Effects of C. helmsii on spawning and hatching
Our study found a negative effect of C. helmsii dominance on spawning site selection and hatching success. Spawning did not take place at random. Sites overgrown by the invasive plant species were clearly avoided for spawning. Instead, E. calamita selected sites with sparsely vegetated or bare soils. The species’ preference of spawning in bare locations is confirmed by several other studies in Europe (e.g., Lopéz-Jurado, 1983; Sinsch, 1998; Pujol-Buxó et al., 2019; Smith, 2019) and specifically pointed out for the Netherlands (Bij12, 2017). It remains unclear whether E. calamita stops spawning when the waterbody is completely dominated by the invasive plant species, especially because several authors report of spawning in densely vegetated pools (Reyne et al., 2021, personal observations by W. de Vries in Denmark, M. van de Loo in Spain, France and Poland, J. van der Loop in the Netherlands, and R. Rannap in Estonia and Denmark). However, our results also show that hatching success of eggs under C. helmsii dominance is lower. Our observed dampening of microclimatic daily temperature fluctuations caused by C. helmsii inside as well as outside enclosures is a plausible explanation for this phenomenon. High irradiation and resulting higher daytime water temperatures are known to be beneficial for egg development (Banks & Beebee et al., 1988; Pujol-Buxó et al., 2019) and survival (Sanuy et al., 2008). Therefore, we conclude that high C. helmsii cover can pose a threat to the survival of E. calamita populations.
Effects of C. helmsii on larval growth
Contrastingly, larval growth and onset of metamorphosis were considerably faster under C. helmsii dominance than on bare soils. It is unclear why tadpoles accelerated their growth under C. helmsii dominance. One possible explanation is that the higher initial density of tadpoles in bare soil enclosures (due to higher hatching success) resulted in higher competition, ultimately affecting growth rate (e.g., Heusser, 1972; Tejedo & Reques, 1994). However, incorporating the initial number of tadpoles present in the GAM formulations did not result in a significant improvement in model fit, suggesting that density effects on larval growth were small in our experiment. This is in agreement with Beebee (1979) and Brady & Griffiths (2000), who concluded that E. calamita shows low sensitivity to intraspecific competition. Additionally, in natural situations higher densities of larvae are no exception (e.g., Sinsch, 1998). We also hypothesized that high nutrient uptake by the invasive plant would reduce the growth of algae, thus reducing availability of the most important food source for tadpoles. This was clearly not the case since dissolved nutrients were lower in enclosures dominated by C. helmsii but this did not result in reduced growth of tadpoles. An alternative explanation is that the structure of C. helmsii leaves creates an enlarged substrate for algae and thereby increases the availability of algal food. Since we did not measure algal biomass in the water column nor the biomass of algae present on plant tissue, this hypothesis requires further investigation.
Another explanation of changes in larval growth in C. helmsii dominated enclosures concerns multiple significant effects of C. helmsii on physical and chemical properties of the water. Evaporation and drying up of the lake had a strong concentrating effect on various dissolved chemicals. The effect of pH during the last two measurements might have resulted from decomposition of organic matter at higher day temperatures. However, this occurred at the end of the experiment, well after tadpoles had been able to grow into the metamorph stage. Low pH and/or high concentration of ammonium and aluminium can have a lethal effect on E. calamita tadpoles (Leuven et al., 1986; Sinsch, 1998; Fedorenkova et al., 2012). However, all concentrations of measured water chemistry parameters were within ranges of optimal breeding sites of E. calamita in Northern Europe (SI Table 11). When comparing bare soil and C. helmsii enclosures, C. helmsii did significantly affect several water chemistry parameters. C. helmsii reduced pH slightly (from 6.3 to 6.0), probably because of residual products originating from storage and breakdown of malic acid during CAM-photosynthesis of C. helmsii. This is beneficial for this plant species as CO2 concentrations are higher at a lower pH, and the plant is unable to use HCO3− for photosynthesis (Newman & Raven 1995). However, pH did not decrease to values that could have significantly affected larval fitness. E. calamita is able to survive in waters with a pH ranging from 4.3 to 8.6 (Sinsch, 1998), and the lethal pH for tadpoles is below 4.3 (Leuven et al., 1986; Federenkova et al., 2012).
Concentrations of ammonium were lower in enclosures dominated by C. helmsii. Inorganic nitrogen was most probably reduced due to uptake by plants for growth as well. Effects on E. calamita are not expected as this species is known to tolerate the measured maximum NH4+ concentrations. Measured pH values were circumneutral and could therefore not add to ammonium toxicity. Other studies also failed in establishing a relationship between ammonium and growth or development of tadpoles (Bregulla, 1986; Dannefelser & Sinsch 1993; Federenkova et al., 2012). However, the production of algae, which depends on inorganic nitrogen availability (among other parameters), could be altered resulting in a different composition of algae species and biomass of algae available for tadpoles (e.g., Smith, 1983a, b; Piorreck et al., 1984).
During the experiment, water in the enclosures started to evaporate resulting in higher concentrations of ions, as is apparent from the increase in inert sodium and chloride. The increase in concentrations of sodium and chloride are not expected to negatively affect tadpoles of E. calamita as they successfully survive in habitats with broader ranges of these ions (Sinsch, 1998).
Effects of differences in water temperature on larval growth (Golay, 1996; Sinsch, 1998) could also not provide a satisfactory explanation for the increased larval growth in C. helmsii dominated enclosures. Scaled fluctuation was lower in C. helmsii dominated enclosures compared to bare soil enclosures. For eggs, this microclimatic dampening effect of C. helmsii is the most plausible explanation for reduced egg hatching success. For tadpoles, this is apparently less important. High temperature extremes in bare soil treatment may have resulted in increased temperature stress for tadpoles. However, mean temperature maxima as well as daily fluctuations were markedly higher outside the enclosures (possibly due to shading effects of wall and the covering mesh of the enclosures), undermining this explanation. Other studies on E. calamita have shown that higher water temperatures result in accelerated growth due to accelerated metabolism (Smith-Gill et al., 1979; Sanuy et al., 2008,). However, these authors only refer to the average temperature over 24 h, and diurnal temperature fluctuations are not included. In our experiment, average water temperature in C. helmsii dominated enclosures did not differ from the bare soil treatment, and was comparable to those encountered outside the enclosures. Temperature maxima in bare soil enclosures were higher and minima were lower in C. helmsii dominated enclosures. During the experiment, tadpoles in C. helmsii dominated enclosures were observed to mainly graze on top of the vegetation, directly below the water surface, probably enabling the tadpoles to increase their exposure to solar irradiation. Tadpoles in bare soil enclosures were observed to mainly graze on the bottom of the enclosure, and not on the water surface of the enclosure. Thus, tadpoles were able to select for their preferred temperature environment in both treatments, resulting in two distinct behavioural choices. Such sun exposed enclosures with warmer water, will probably optimize temperature for grazing and growth of larvae.
The lower water level observed in enclosures containing bare soils might explain the smaller size attained by tadpoles in these enclosures. Water levels differed between treatments because enclosures placed on soils dominated by C. helmsii were on average located deeper in the waterbody. The combined effect of changes in water level and vegetation may have caused the difference in larval development speed. Water levels and the speed of drying-out influenced larval development (e.g. Tejedo & Reques, 1994; Brady & Griffiths, 2000). Tadpoles of E. calamita accelerate their development during desiccation of a water body, resulting in smaller larvae entering metamorphosis. The drying out coincided with a shorter body length of tadpoles reaching metamorphosis, but did not lead to faster larval development in the bare soil treatment.
Thus, we conclude that larval growth was significantly higher in C. helmsii dominated enclosures than on bare soil, but that differences in measured abiotic conditions cannot explain this phenomenon. We hypothesize that algal food could be higher in C. helmsii treatments, as the leaves and stems of the plant increase the surface area for algae growth and thus availability of algae. To what extent this benefits the tadpoles warrants further research.
Limitations of the study
The experiment did not include a control with enclosures dominated by native plant species instead of an alien invader as native vegetation was absent at our study site. One major consequence of this lack of a positive control is that we cannot prove that similar effects on E. calamita are found when ponds are increasingly populated by native vegetation, hence reducing bare ground and increasing vegetation cover. Since the location was managed to maintain a bare soil environment, effects of high native vegetation cover could not been assessed in past nor future. The rapid growth of invasive C. helmsii renders this management goal increasingly difficult to reach. However, since Reyne et al. (2021) found that spawning of E. calamita was associated with a high percentage cover of aquatic vegetation in lakes, but with short terrestrial vegetation in the surrounding vicinity, it may be possible that egg deposition and successful reproduction are not hindered by an increase of vegetation.
Unfortunately, we were not able to maintain equal water levels between different treatment groups over the course of the experiment, as the bare soil treatments dried out faster because of shallower placement. This was an unforeseen uncontrolled small-scaled effect of the locations as temperatures and water evaporation were higher than expected for this time of year. Consequence is that the measured differences were due to placement rather than treatment.
Conducting a field study can always yield unexpected variables. However, we do not feel that performing this experiment in a controlled laboratory environment is justified due to the declining population in this ‘De Gijzenrooise Zegge’ of this protected amphibian, and various infectious disease risks associated with transport of egg strings, tadpoles and juveniles. By conducting the experiment at the field location, the population of E. calamita experienced as little disturbance as possible. In addition, variables that are natural but cannot be simulated in the laboratory are available thanks to implementation in the field. Conducting the study in the field may more accurately reflect biological reality, due to the incorporation of these additional variables. However, we think a laboratory version to have other, comparable consequences.
Demographic implications
C. helmsii alters demographic parameters of E. calamita populations, since it significantly reduces egg spawning and hatching success where it is dominant, i.e. with a dense vegetation cover of 95–100%. Number of egg strings is positively correlated with number of tadpoles (Reyne et al., 2021), and lower egg production results in a lower reproduction success. Although counting the number of egg strings cannot be used to directly calculate population size (Schmidt, 2004, 2005), it serves as a time efficient indication of the relative change in the number of females spawning over time. It remains unclear whether nature areas become completely unsuitable for reproduction of E. calamita when C. helmsii dominates an area, causing this species to become locally extinct. In cases of low reproduction rate and deteriorated habitat quality, local extinction of populations of E. calamita can quickly occur (Zahn et al., 2020). However, growth is accelerated in our enclosures under C. helmsii dominance. This can be beneficial for E. calamita as larger tadpoles at metamorphosis, with wider heads and thicker hind legs, may exhibit increased feeding, fleeing and dispersion capability, resulting in increased survival as toads (Tejedo et al., 2010). Additionally, a larger size at metamorphosis may increase reproductive success as larger males are more attractive and larger females can produce bigger clutch and eggs (Tejedo 1992a,b). Our experiments could however also simply indicate that tadpoles prefer aquatic vegetated patches over bare soil. In this case, heterogenic shallow water habitats containing bare soil areas for egg development as well as vegetated areas for larval development are probably most suitable as breeding sites for E. calamita, similar to findings of Reyne et al., 2021. Without control measures however, invasive C. helmsii completely overgrows these habitats, and patches of bare soil, and the presence of standing water, will quickly disappear (SI Fig. 8). Additionally, in North-western Europe, shallow lakes similar to our study site are normally characterized by native plant species of the Littorellion uniflorae plant association. The growth forms of these species (i.e. Littorella uniflora (L.) Rusby, Pilularia globulifera L. and Baldellia ranunculoides subsp. repens (Lam.) Á.Löve & D.Löve) remarkably differ from that of C. helmsii (a low-growing vegetation with isoetid habit that grows slowly versus faster biomass production and higher plant growth of the invader) (Den Hartog & Segal, 1964; Bloemendaal & Roelofs, 1988; Dawson & Warman; 1987). C. helmsii causes different soil conditions, i.e. because of altered light penetration, and therefore will differentially affect spawning behaviour of E. calamita.
All things considered, this study still leaves considerable uncertainty about the demographic implications for E. calamita when C. helmsii is present. However, the negative effect on spawning and hatching success, in combination with the high impact of C. helmsii infestation on vegetation structures (e.g., Dawson & Warman, 1987; Leach and Dawson, 1999; Van Kleef et al., 2017; Smith & Buckley, 2020) justifies erring on the side of caution and we recommend to implement management of C. helmsii in breeding habitats of E. calamita. Future research and demographic modelling of E. calamita reproduction in breeding sites recently infected by C. helmsii will assist in elucidating the ultimate effects on population viability of E. calamita. In such research, special attention should be paid to further unravel the causal mechanisms determining the speed of larval growth under C. helmsii dominance, not only relating to availability of bare soil but also to varying cover of native vegetation. In addition, it is recommended to determine the effects of C. helmsii on other amphibian species.
Implications for invasive species management for biological conservation
Our research clearly shows that C. helmsii negatively affects spawning and hatching success. On the other hand, a faster growth rate of tadpoles is observed at soils dominated by C. helmsii. This indicates that the population is possibly not negatively affected by the presence of both bare soils and C. helmsii dominated soils. However, in north-western Europe, suitable habitats of both species are almost identical which poses a threat to E. calamita when massive overgrowth of its breeding sites by C. helmsii occurs. The plant species has a tendency to completely suffocate a waterbody by its unlimited growth and can make waterbodies dry out within a few years making waterbodies unsuitable for E. calamita without management (e.g. Smith & Buckley 2020; Van der Loop et al., 2019, 2020). This is expected in the study area as the plant species has only recently spread in the area and expansion of C. helmsii is still ongoing.
An increased effort will be required to protect this Annex IV Habitat Directive species and its habitats as populations of E. calamita are declining due to a shortage of suitable breeding waters and insufficient nature management, among other causes (Husté et al., 2006; Stevens & Baguette, 2008; Goverse, 2009; Beebee et al., 2012). C. helmsii will probably be contributing to this decline given its rapid range expansion and dominance of many freshwater pioneer habitats. Even though eradication of C. helmsii is difficult and very costly (Van der Loop et al., 2018, 2022), measures to prevent spread and population control of this invasive alien plant species need to be taken into account in management of breeding sites of E. calamita.
Data availability
All data is available.
Code availability
Not applicable.
References
Altwegg, R. & H. U. Reyer, 2003. Patterns of natural selection on size at metamorphosis in water frogs. Evolution 57(4): 872–882.
Banks, B. & T. J. C. Beebee, 1988. Reproductive success of natterjack toads Bufo calamita in two contrasting habitats. The Journal of Animal Ecology 57: 475–492.
Bates, D., M. Maechler, B. Boler & S. Walker, 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1): 1–48.
Beebee, T. J., 1979. A review of scientific information pertaining to the natterjack toad Bufo calamita throughout its geographical range. Biological Conservation 16(2): 107–134.
Beebee, T. J., C. Cabido, C. Eggert, I. G. Mestre, A. Iraola, I. Garin-Barrio, R. A. Griffiths, C. Miaud, N. Oromi, D. Sanuy, U. Sinsch & M. Tejedo, 2012. 40 years of Natterjack toad Conservation in Europe. FrogLog 101: 40–44.
Beja, P., Kuzmin, S., Beebee, T., Denoël, M., Schmidt, B., Tarkhnishvili, D., Ananjeva, N.B., Orlov, N.L., Nyström, P., Ogrodowczyk, A., Ogielska, M., Bosch, J., Miaud, C., Tejedo, M., Lizana, M. & I. Martínez-Solano, 2009. Epidalea calamita. The IUCN Red List of Threatened Species 2009: e.T54598A11160828. https://doi.org/10.2305/IUCN.UK.2009.RLTS.T54598A11160828.en.
Bij12, 2017. Kennisdocument Rugstreeppad Bufo calamita. Versie 1.0, juli 2017. (In Dutch).
Blackburn, T. M., C. Bellard & A. Ricciardi, 2019. Alien versus native species as drivers of recent extinctions. Frontiers in Ecology and the Environment 17(4): 203–207.
Bloemendaal, F. H. J. L. & J. G. M. Roelofs, 1988. Waterplanten en waterkwaliteit, Koninklijke Nederlandse Natuurhistorische Vereniging, Utrecht: (In Dutch).
Brady, L. D. & R. A. Griffiths, 2000. Developmental responses to pond desiccation in tadpoles of the British anuran amphibians (Bufo bufo, B. calamita and Rana temporaria). Journal of Zoology 252(1): 61–69.
Bregulla, D., 1986. Untersuchungen Zur Wasserchemie Von Kreuzkröten-Laichgewässern. Salamandra 22(2–3): 173–179. (In German)
Brockelman, W. Y., 1969. An analysis of density effects and predation in Bufo americanus tadpoles. Ecology 50(4): 632–644.
Brouwer, E., L. Denys, E. C. H. E. T. Lucassen, M. Buiks & T. Onkelinx, 2017. Competitive strength of Australian swamp stonecrop (Crassula helmsii) invading moorland pools. Aquatic Invasions 12: 321–331.
Butchart, S. H., M. Walpole, B. Collen, A. Van Strien, J. P. Scharlemann, R. E. Almond & R. Watson, 2010. Global biodiversity: indicators of recent declines. Science 328(5982): 1164–1168.
Calef, G. W., 1973. Natural mortality of tadpoles in a population of Rana aurora. Ecology 54(4): 741–758.
Clavero, M. & E. García-Berthou, 2005. Invasive species are a leading cause of animal extinctions. Trends in Ecology & Evolution 20(3): 110.
Cockerill, D., 1979. Crassula Helmsii. BSBI News 21: 19.
Council of the European Communities, 1992. Council directive 92 /43 /EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Official Journal of the European Communities L206: 7–50.
Creemers, R.C.M., Arntzen, J.W., van Delft, J.C.W. & B. Teunis, 2009. De amfibieën en reptielen van Nederland. Nationaal Natuurhistorisch Museum Naturalis, Leiden. 476 p. (In Dutch)
Dannefelser, B. & U. Sinsch, 1993. Does water volume affect growth, timing of metamorphosis and size of metamorphs in solitary Bufo calamita tadpoles. Zoologisches Institut, Salzburg 86: 223.
Dawson, F. & E. Warman, 1987. Crassula helmsii (T. Kirk) Cockayne: is it an aggressive alien aquatic plant in Britain? Biological Conservation 42: 247–272.
Dean, C. E., J. Day, R. E. Gozlan & A. Diaz, 2015. Grazing vertebrates promote invasive Swamp stonecrop (Crassula helmsii) abundance. Invasive Plant Science and Management 8: 131–138.
Den Hartog, C. & S. Segal, 1964. A new classification of the water–plant communities. Acta Botanica Neerlandica 13(3): 367–393.
Diaz, A., 2012. Crassula helmsii (T.Kirk) Cockayne (New Zealand Pygmyweed). In Francis, R. A. (ed), A handbook of global freshwater invasive species Routledge, Oxford: 37–46.
Ewald, N.C., 2014. Crassula helmsii in the New Forest – A report on the status, spread and impact of this non-native invasive plant, and the efficacy of novel control techniques following a 2 year trial. Partner Annex Report for RINSE prepared on behalf of the New Forest Non-Native Plants Project. Freshwater Habitats Trust, Oxford.
Extended R package version 1.9.0. URL: https://CRAN.R-project.org/package=PMCMRplus Accessed 7 February 2021.
Fedorenkova, A., J. A. Vonk, H. R. Lenders, R. C. Creemers, A. M. Breure & A. J. Hendriks, 2012. Ranking ecological risks of multiple chemical stressors on amphibians. Environmental Toxicology and Chemistry 31(6): 1416–1421.
Fox, J. & S. Weisberg, 2019. An {R} Companion to Applied Regression, Third Edition. Thousand Oaks CA: Sage. https://socialsciences.mcmaster.ca/jfox/Books/Companion/ Accessed 7 February 2021.
Gastwirth, J.L., Gel, Y.R., Hui, W.W.L., Lyubchich, Miao, V.W. & K. Noguchi, 2020. lawstat: Tools for Biostatistics, Public Policy, and Law. R package version 3.4. https://CRAN.R-project.org/package=lawstat Accessed 7 February 2021.
Genovesi, P., 2005. Eradications of invasive alien species in Europe: a review. Biological Invasions 7(1): 127–133.
Golay, N., 1996. Die Kreuzkröte (Bufo calamita Laur.) als Pionierart. PhD-thesis. Medizinische Biologie Institut für Pathologie. University Basel, Basel. (In German)
Goverse, E., 2009. Zorgen om de rugstreeppad. Stichting RAVON in Nature today. https://www.naturetoday.com/intl/nl/nature-reports/message/?msg=16441 Accessed 7 February 2021. (In Dutch)
Grutters, B. M., B. J. Pollux, W. C. Verberk & E. S. Bakker, 2015. Native and non-native plants provide similar refuge to invertebrate prey, but less than artificial plants. PLoS ONE 10(4): e0124455.
Harrell, F.E. & C. Dupont, 2020. Harrell Miscellaneous. R package version 4.4–2. https://CRAN.R-project.org/package=Hmisc Accessed 7 February 2021.
Heusser, H., 1972. Intra-und interspezifische Crowding-Effekte bei Kaulquappen der Kreuzkröte Bufo Calamita Laur. Oecologia 10(1): 93–98. (In German)
Hobbs, R. J. (ed), 2000. Invasive Species in a Changing World. Island Press, Washington.
Hothorn, T., F. Bretz & P. Westfall, 2008. Simultaneous inference in general parametric models. Biometrical Journal 50(3): 346–363.
Hussner, A., 2009. Growth and photosynthesis of four invasive aquatic plant species in Europe. Weed Research 49(5): 506–515.
Husté, A., J. Clobert & C. Miaud, 2006. The movements and breeding site fidelity of the natterjack toad (Bufo calamita) in an urban park near Paris (France) with management recommendations. Amphibia-Reptilia 27(4): 561–568.
Kamphake, L. J., S. A. Hannah & J. M. Cohen, 1967. Automated analysis for nitrate by hydrazine reduction. Water Research 1(3): 205–216. https://doi.org/10.1016/0043-1354(67)90011-5.
Kleiber, C. & A. Zeileis, 2008. Applied Econometrics with R, Springer, New York:
Langdon, S. J., R. H. Marrs, C. A. Hoise, H. A. McAllister, K. M. Norris & J. A. Potter, 2004. Crassula helmsii in U.K. ponds: effects on plant biodiversity and implications for newt conservation. Weed Technology 18: 1349–1352.
Leach, J. & H. Dawson, 1999. Crassula helmsii in the British Isles-an unwelcome invader. British Wildlife 10: 234–239.
Leuven, R. S. E. W., C. den Hartog, M. M. C. Christiaans & W. H. C. Heijligers, 1986. Effects of water acidification on the distribution pattern and the reproductive success of amphibians. Experientia - Cellular and Molecular Life Sciences 42(5): 495–503.
Lockton, A.J., 2009. Crassula helmsii. London, UK: Botanical Society of the British Isles. http://sppaccounts.bsbi.org.uk/content/crassula-helmsii-2 Accessed 25 September 2022.
López-Jurado, L. F., 1983. Estudios sobre el sapo corredor (Bufo calamita) en el sur de España III. Reproducción. Doñana. Acta Vertebrata 9: 53–69. (In Spanisch)
McNeely, J., 2001. Invasive species: a costly catastrophe for native biodiversity. Land Use and Water Resources Research 2: 1–10.
Mooney, H. A. & E. E. Cleland, 2001. The evolutionary impact of invasive species. Proceedings of the National Academy of Sciences 98(10): 5446–5451.
Ministry of Agriculture, Nature and Food Quality, 2009. Rode lijsten: soort van Rode Lijst Amfibieën. Rugstreeppad, Bufo calamita. Staatscourant 2009, 13201. https://minez.nederlandsesoorten.nl/content/rode-lijsten-soort-van-rode-lijst-amfibie%C3%ABn Accessed on 2 March 2021. (In Dutch)
Netherlands Enterprise Agency, 2014. Rugstreeppad Bufo calamita Soortenstandaard. Rijksdienst voor ondernemend Nederland, Utrecht (In Dutch).
Newman, R. A., 1988. Adaptive plasticity in development of Scaphiopus couchii tadpoles in desert ponds. Evolution 42(4): 774–783.
Newman, J.R., 2013. CEH Information Sheet 12: Crassula helmsii, Australian Swamp Stonecrop. Centre for Ecology & Hydrology, CAPM, CEH Wallingford, Crowmarsh Gifford, Wallingford, Oxon, OX10 8BB, 3, 1 p.
Newman, J. R. & J. A. Raven, 1995. Photosynthetic carbon assimilation by Crassula helmsii. Oecologia 101(4): 494–499.
OEPP, EPPO, 2007. Data sheets on quarantine pests. Crassula helmsii. EPPO European and Mediterranean Plant Protection Organization 37: 225–229.
Pilkington, S., 2016. Channelled crystalwort Riccia canaliculata in England. Field Bryology 116: 6–9.
Piorreck, M., K. H. Baasch & P. Pohl, 1984. Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regimes. Phytochemistry 23(2): 207–216.
Pohlert, T., 2021. “PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums Extended,” R Package Version 1.4.4, https://CRAN.R-project.org/package=PMCMRplus. [3] Accessed 7 February 2021.
Prinz, M., C. Peppler-Lisbach, A. Weidhüner & H. Freund, 2019. Crassula helmsii (T. Kirk) Cockayne: Standortansprüche, Verbreitung und Vergesellschaftung eines invasiven Neophyten auf Norderney. Tuexenia 39: 267–286. (In German)
Pujol-Buxó, E., G. M. Riaño & G. A. Llorente, 2019. Mild segregation in the breeding preferences of an invasive anuran (Discoglossus pictus) and its main native competitor (Epidalea calamita) in ephemeral ponds. Amphibia-Reptilia 1: 1–11.
Rabitsch, W., Aronsson, M., Strand, M. & S. Roscher, 2020. Impact caused by Invasive Alien Species of Union concern on habitats and species of the Nature Directives and Natura 2000 sites. ETC/BD Technical paper 3/2020. European Topic Centre on Biological Diversity, Paris. 80 p.
Reques, R. & M. Tejedo, 1997. Reaction norms for metamorphic traits in natterjack toads to larval density and pond duration. Journal of Evolutionary Biology 10(6): 829–851.
Reyne, M., A. Aubry, M. Emmerson, F. Marnell & N. Reid, 2021. Conservation efforts fail to halt the decline of the regionally endangered Natterjack toad (Epidalea calamita) in Ireland. Biological Conservation 261: 109–228.
Rodriguez, L. F., 2006. Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur. Biological Invasions 8(4): 927–939.
Ruckelshaus, M. H., S. T. Jackson, H. A. Mooney, K. L. Jacobs, K. A. S. Kassam, M. T. Arroyo, A. Báldi, A. M. Bartuska, J. Boyd, L. N. Joppa & A. Kovács-Hostyánszki, 2020. The IPBES global assessment: pathways to action. Trends in Ecology & Evolution 35(5): 407–414.
Sanuy, D., N. Oromí & A. Galofré, 2008. Effects of temperature on embryonic and larval development and growth in the natterjack toad (Bufo calamita) in a semi-arid zone. Animal Biodiversity and Conservation 31(1): 41–46.
Schmidt, B. R., 2004. Declining amphibian populations: the pitfall of count data in the study of diversity, distribution, dynamics and demography. Herpetol. J. 14: 167174.
Schmidt, B. R., 2005. Monitoring the distribution of pond-breeding amphibians when species are detected imperfectly. Aquatic Conservation 15: 681–692.
Sinsch, U., 1998. Biologie und Ökologie der Kreuzkröte: Bufo calamita. Laurenti-Verlag, plaats uitgave
Smith, D. C., 1983a. Factors controlling tadpole populations of the chorus frog (Pseudacris triseriata) onIsle Royale. Michigan. Ecology 64(3): 501–510.
Smith, V. H., 1983b. Low nitrogen to phosphorus ratios favor dominance by blue-green algae in lake phytoplankton. Science 221(4611): 669–671.
Smith, T., 2015. The environmental impact of Crassula helmsii. PhD thesis, Canterbury Christ Church University, Canterbury.
Smith, T. & P. Buckley, 2015. The growth of the non-native Crassula helmsii (Crassulaceae) increases the rarity scores of aquatic macrophyte assemblages in south-eastern England. New Journal of Botany 5(3): 192–199.
Smith, T. & P. Buckley, 2020. Biological Flora of the British Isles: Crassula helmsii. Journal of Ecology 108(2): 797–813.
Smith, P. H. & G. Skelcher, 2019. Effects of environmental factors and conservation measures on a sand-dune population of the natterjack toad (Epidalea calamita) in north-west England: a 31-year study. Herpetological Journal 29(3): 146–154.
Smith-Gill, S. J. & K. A. Berven, 1979. Predicting amphibian metamorphosis. The American Naturalist 113(4): 563–585.
Spiess, A.N., 2018. qpcR: Modelling and Analysis of Real-Time PCR Data. Rpackage version 1.4–1. https://CRAN.R-project.org/package=qpcR Accessed 7 February 2021.
Stevens, V. M. & M. Baguette, 2008. Importance of habitat quality and landscape connectivity for the persistence of endangered natterjack toads. Conservation Biology 22(5): 1194–1204.
Swale, E. & H. Belcher, 1982. Crassula helmsii, the swamp stonecrop, near Cambridge. Nature in Cambridgeshire 25: 59–62.
Team RC, 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. 2012. http://www.R-project.org Accessed 7 February 2021.
Tejedo, M., 1992a. Effects of body size and timing of reproduction on reproductive success in female natterjack toads (Bufo calamita). Journal of Zoology 228(4): 545–555.
Tejedo, M., 1992b. Large male mating advantage in natterjack toads, Bufo calamita: sexual selection or energetic constraints? Animal Behaviour 44: 557–569.
Tejedo, M. & R. Reques, 1994. Plasticity in metamorphic traits of natterjack tadpoles: the interactive effects of density and pond duration. Oikos 71: 295–304.
Tejedo, M., F. Marangoni, C. Pertoldi, A. Richter-Boix, A. Laurila, G. Orizaola, A. G. Nicieza, D. Álvarez & I. Gomez-Mestre, 2010. Contrasting effects of environmental factors during larval stage on morphological plasticity in post-metamorphic frogs. Climate Research 43(1–2): 31–39.
Van der Loop, J. M. M., L. de Hoop, H. H. van Kleef & R. S. E. W. Leuven, 2018. Effectiveness of eradication measures for the invasive Australian swamp stonecrop Crassula helmsii. Management of Biological Invasions 9(3): 343–355.
Van der Loop J.M.M, Beringen R., Leuven R.S.E.W., Van Valkenburg J.L.C.H., Van Kleef H.H., Verhofstad M., B. Odé, 2019. Risk assessment of Australian swamp stonecrop (Crassula helmsii) in Europe FLORON report: 2019.064.
Van der Loop, J. M. M., J. Tjampens, J. J. Vogels, H. H. van Kleef, L. P. Lamers & R. S. E. W. Leuven, 2020. Reducing nutrient availability and enhancing biotic resistance limits settlement and growth of the invasive Australian swamp stonecrop (Crassula helmsii). Biological Invasions 11: 391–402.
Van der Loop, J.M.M., van de Loo, M., de Vries, W., van Veenhuisen L.S., van Kleef, H.H. & R.S.E.W. Leuven, 2022. Lessons learnt from large-scale eradication of Australian swamp stonecrop Crassula helmsii in a protected Natura 2000 site Management of Biological Invasions, 13, in press.
Velle, G., H. Skoglund & B. T. Barlaup, 2022. Effects of nuisance submerged vegetation on the fauna in Norwegian rivers. Hydrobiologia 849(2): 539–556.
Watson, W., 2001. An unwelcome aquatic invader. Worcestershire Record. http://www.wbrc.org.uk/worcrecd/Issue10/invader.htm Accessed 7 February 2021.
Wood, S. N., 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society (b) 73(1): 3–36.
Wood, S. & F. Scheipl, 2020. gamm4: Generalized Additive Mixed Models using 'mgcv' and 'lme4'. R package version 0.2–6. https://CRAN.R-project.org/package=gamm4 Accessed 7 February 2021.
Zahn, A., B. Pelikofer & J. Späth, 2020. Stirb langsam? Aussterbevortgänge bei Wechselkröte (Bufotes viridis) und Kreuzkröte (Epidalea calamita). (Die slow? Extrinction processes in green toad (Bufotes viridis) and natterjack toad (Epidelea calamita). Herpetologie 27: 229–238. (In German)
Acknowledgements
This study was financially supported by the subsidy ‘Biodiversiteit en Leefgebieden’ [grant code C2224865/4367096] from the Province of Noord-Brabant, and a contribution of the municipality Eindhoven [Grand Code 3635146/9219000098] both located in The Netherlands. Furthermore, we are grateful to the nature managers of Stichting Brabants Landschap from our research site for allowing field studies. The authors would like to thank the staff of Stichting Bargerveen, Soontiëns Ecology and Radboud University for their advice and technical assistance during the experiment and the execution of management measures. We thank Samuel Tasker MSc. for the English proofreading. We thank Serge Bogaerts MSc., Julian Brouwer BSc., Prof. dr. Frank Pasmans, the editor Prof. dr Andre Padial and two anonymous reviewers for their constructive comments on the manuscript.
Funding
This study was financially supported by the subsidy ‘Biodiversiteit en Leefgebieden’ [Grant Code C2224865/4367096] from the Province of Noord-Brabant, and a contribution of the municipality Eindhoven [Grand Code 3635146/9219000098] both located in The Netherlands.
Author information
Authors and Affiliations
Contributions
JMMvdL: Conceptualization, methodology, data curation, formal analysis, investigation, writing—Original draft, writing. LSvV: data curation, formal analysis, visualization, writing—review & editing. MvdL: conceptualization, methodology, investigation, writing—review & editing. JV: data curation, formal analysis, visualization, writing—review & editing. HHvK: conceptualization, methodology, investigation, project administration, writing—review & editing. Supervision RSEWL: conceptualization, writing—review & editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no known competing interests, financial or otherwise that have influenced the contents of this paper.
Additional information
Handling editor: Andre A. Padial
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Loop, J.M.M., van Veenhuisen, L.S., van de Loo, M. et al. Invasive Australian swamp stonecrop (Crassula helmsii) negatively affects spawning but accelerates larval growth of the endangered natterjack toad (Epidalea calamita). Hydrobiologia 850, 699–714 (2023). https://doi.org/10.1007/s10750-022-05117-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05117-y