Abstract
The regression of fisheries in the Gulf of Cadiz is evident since current fish catches are 33% of that 30 years before. Consequently, some initiatives for the replenishment of exhausted wild stocks are welcome. The objective of the present work is to describe and analyse the results coming from the first flatfish stock enhancements in Andalusia. A total of 3189 fish from three flatfish species: Senegal sole (Solea senegalensis Kaup), wedge sole (Dicologlossa cuneata Moreau), and brill (Scophthalmus rhombus Linnaeus) were tagged and released. Several variables were calculated through the data analysis of recovered fish. Some variables were calculated only for Senegal soles since wedge sole and brill recaptures were not significant. The Senegal sole recapture rate was 2.71 ± 0.72, similar to other published data, the recapture rates for bigger fish being higher though not significant. No significant differences were detected for distance, time, growth, or recapture rate amongst initial Senegal sole sizes. Around 80% of recaptures were registered within 15 weeks after release. The results show that it is possible for the release and recovery of tagged Senegal soles in the Gulf of Cadiz. Future long-term programmes on stock enhancement could help to determine the effects on fisheries and recover stocks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish stock enhancement is aimed at several different purposes though all of them focus on the reinforcement of the wild stocks, for both productive (fisheries enhancement) and ecological (ecosystem and/or species protection) targets. In this way, releasing hatchery-produced (cultured) fish juveniles in the wild can improve the system productivity through different ways: replenishing severely depleted fisheries, enhancing the production of recruitment-limited fisheries, and aggregating/relocating adults for some species (Bell et al., 2006).
The first stock enhancement programmes took place in Japan (1963) and Norway (1999). Currently the former has released 72 species of fish, shellfish, and other invertebrates, supposing 2000 millions of released fish, mainly chum salmon (Oncorhynchus keta Walbaum) (Kitada et al., 1992; Ishino, 1999; Yamashita & Yamada, 1999; Kitada, 2018). Norway has also developed several restocking programmes with some species, mainly Atlantic salmon (Salmo salar Linnaeus) and cod (Gadus morhua Linnaeus) (Svåsand et al., 2000; Andersen et al., 2005; Støttrup & Sparrevohn, 2007; Hagen et al., 2020). In fact, fish stock enhancement is an usual activity involved in long-term programmes and several works have reported its beneficial effects on the wild stocks of some species as the barfin flounder (Verasper moseri Jordan & Gilbert), the spotted halibut Verasper variegatus (Temminck & Schlegel), the Japanese sea bream (Pagrus major Temminck & Schlegel) and flounder (Paralichthys olivaceus Temminck & Schlegel), the flathead grey mullet (Mugil cephalus Linnaeus), and the red drum (Sciaenops ocellatus Linnaeus) (Rutledge, 1989; Leber & Arce, 1996; Kitada & Kishino, 2006; Wada et al., 2012, 2014).
In addition to Japanese and barfin flounders and spotted halibut, other stock enhancement programmes with flatfish have been carried on 1990–2004 in Denmark. Concretely, nearly one million of turbots (Scophthalmus maximus Linnaeus) have released in several places from the Danish coast (Støttrup et al., 2002; Paulsen & Støttrup, 2004; Sparrevohn & Støttrup, 2007). Thanks to those works, the authors have reported that some variables such as the depth distribution, growth, and nutritional status were similar between released and wild turbots. Hence, they have stated that this species is robust and unaffected by release procedures and handing and, consequently, the release of hatchery-produced turbots can increase the fishery recruitment.
Therefore, the fish stocking activities have usually contributed to the protection of fish stocks and the maintaining of a sustainable development of coastal fisheries (Chen et al., 2015). Nevertheless, Kitada (2018) has reported that a general lack of evaluation of positive and negative effects of hatchery releases exist, despite stocking activities are economically profitable in the most of cases. For this reason, many works continue publishing focused on the study and reduction of genetic and ecological impacts, and the improvement of the fish tagging methods and the fisheries assessment (Berejikian et al., 2012; Christie et al., 2012; Nakajima et al., 2013; González et al., 2015; Szuwalski et al., 2015; Bolstad et al., 2017; Grant et al., 2017; Yang et al., 2017).
In the Southern Spanish coast the first marine fish stock enhancements were carried out in 1993 with gilthead sea bream (Sparus aurata Linnaeus) juveniles (Sánchez-Lamadrid, 2002). Later, this and other fish species were released in that coast: Senegal soles (Solea senegalensis Kaup), white seabreams (Diplodus sargus Linnaeus), red-banded sea breams (Pagrus auriga Valenciennes), red sea breams (Pagrus pagrus Linnaeus), and rubberlip grunts (Plectorhinchus mediterraneus Guichenot) (unpublished data). In Spain also turbot juveniles have been released in the Northern coasts (Pérez et al., 2004).
Nevertheless, the most of those activities were not involved in any research project/programme, hence the available information about them is scarce. In fact, our research team also released 10,000 Senegal sole alevins (1–1.5 g body weight) in the Gulf of Cadiz (2 miles from Huelva’s coast) in 2005. These fish were not tagged because of their small size, hence no research on recapture could be carried out. Only Sánchez-Lamadrid (2002, 2004) provided information on size, tags, and other parameters in several releases of gilthead sea breams made in the Bay of Cadiz.
The Senegal sole and wedge sole are very valuable commercial flatfish in the Gulf of Cadiz. These species joint to other soleids have reached a worldwide fishery production of 41,000 MT in 2016, and the main countries are the Netherlands, Nigeria, France, and Morocco (FAO, 2018). They inhabit Eastern Atlantic muddy and sandy bottoms (0–65 m depth), from Senegal to the Iberian Peninsula; being also present in the Western Mediterranean sea (Bauchot, 1987; Ben-Tuvia, 1990). Senegal soles have nocturnal feeding habits and prey upon benthic invertebrates: crustaceans, polychaetes, and molluscs (Garcia-Franquesa et al., 1996). Wedge sole feeding is based on polychaetes, amphipods, and bivalves (Belghyti et al., 1994; Ramos et al., 1996; Cabral et al., 2002). Both species are gonocoric, reaching the sexual maturity at 15 cm (+ 1 year) and 25 cm (+ 3 years) length for wedge and Senegal sole, respectively (Dinis, 1986; Jiménez et al., 1998). The maximum length in adults is 30 and 60 cm for D. cuneata and S. senegalensis, respectively (Desoutter, 1990; Pérez and Rodríguez, 2001). Both soleid species are mainly fished near coast with gillnets and bottom trawls, and the fishery catches in Andalusia are around 17 and 97 MT for S. senegalensis and D. cuneta, respectively (Consejería de Agricultura y Pesca, 2001; Consejería de Agricultura, Pesca y Desarrollo Rural, 2017). In the Gulf of Cadiz, Senegal and wedge soles are fished all year around; their peaks usually being in Feb–Mar and Jan–Feb, respectively. There are no seasonal restrictions for their fishing although they have minimum legal sizes, 24 and 15 cm for Senegal sole and wedge sole, respectively.
Worldwide, the fishery production of brill is around 5700 MT, the Netherlands, France, and the UK being the main producers (FAO, 2018). This species is not abundant in the Gulf of Cadiz although its market value is one of the highest in the Andalusian fish markets (19 € Kg−1). In fact, a small fishery production of 6 MT supposed 127,000 € in 2017, being the second most expensive fish species (Consejería de Agricultura, Pesca y Desarrollo Rural, 2017). It inhabits coastal waters (5–50 m depth) from Eastern Atlantic to the Mediterranean Sea and its feeding is based on fish and big crustaceans. Brills are gonocoric and reach the sexual maturity at 37 cm (around 3 years old) (Bauchot 1987). As the previous species, they are fished all year around in the Gulf of Cadiz, with no seasonal restrictions, the minimum legal size being 30 cm.
The regression of the fisheries in the Gulf of Cadiz (Spanish South-west) is evident since current fish catches are 33% of that 30 years before (Consejería de Agricultura, Pesca y Desarrollo Rural, 2017). Currently, there are no accurate and consistent data on brill and Senegal sole fishery production since they are often mixed with other species in general groups, like “Soles” and “Turbots”. Nevertheless, the wedge sole catches have dropped up to 3% in 30 years. Consequently, some initiatives for the replenishment of close to depletion wild stocks were welcome. Taking this into account, the objective of this work is to describe and analyse the results coming from the first flatfish stock enhancements in Andalusia (Southern Spain).
Materials and methods
This work was directed by trained scientists (category C) and it was carried out in compliance with the Guidelines of the European Union Council (86/609/EU), and the Spanish Government (RD1201/2005) for the use of animals in research.
Zootechnical and transport procedures
Fish were reared at the IFAPA Agua del Pino, sited in Cartaya (South-western Spain). This research centre had several broodstocks from three different commercial flatfish species: Solea senegalensis, Scophthalmus rhombus, and Dicologlossa cuneata. Those spawners were fished from the wild and adapted to captivity during 2002–2005, and the whole broodstock was consisted of 78 Senegal soles, 58 wedge soles, and 32 brills.
Specific culture protocols (from egg to juveniles) for every species were followed in order to get juveniles (Ribeiro et al., 1999; Herrera et al., 2008; Hachero-Cruzado et al., 2009). Briefly, the eggs were collected through natural spawning and incubated at 20 °C (Senegal and wedge soles) and 14 °C (brill) for 2–5 days. After hatching, the larval rearing was carried on 250-l cylinder-conical tanks at 20 °C in a flow-through system. Brill larvae were progressively acclimated to that temperature (1 degree/day increase). The larval feeding consisted of rotifer and Artemia until the weaning at 40–60-day post-hatching. Later fish were fed commercial marine fish feed (Skretting LE-3, Burgos, Spain) according to the manufacturer recommendations (around 1–3% biomass daily). Three weeks before the fish release they were fed commercial frozen natural food (mussels and squid), since it has been proposed that a previous natural food-based diet can increase the stock enhancement efficacy (Walsh et al., 2013).
Fish were starved 24 h prior the release day, and the water culture temperature during the last week was progressively modified to 16 °C, similar to the natural seawater temperature. Fish were transported from IFAPA Agua del Pino to Mazagón’s harbour into 600-l isothermal tanks (89 × 110 × 57 cm) through a refrigerated truck. The water temperature was 16–17 °C and every tank was linked to an oxygen bottle through air-stones in order to keep the water dissolved oxygen above 5 ppm. The stocking density was less than 16 kg m− 2. The tanks were moved out the truck to the ship by a crane. Once tanks were placed on the ship deck, the oxygen supply was stopped and tanks water was renewed through pumping from the sea water, hence fish could be acclimated to the natural water physical–chemical conditions for more than one hour before releasing.
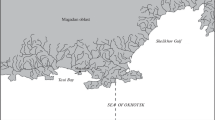
Several batches of tagged flatfish (2010 Senegal soles, 756 wedge soles, and 423 brills) were released in two artificial reefs (El Rompido and Matalascañas) placed in the South-western Spanish coast (see more details in Table 1 and Fig. 1). These places were chosen to ensure an initial shelter for released fish since the most of fishing gears (mainly gillnets) cannot be used in those areas (risk of breaking or hooking).
Tagging, sampling, and data collection
Prior transport and release, all fish were measured (total length and body weight, Table 1) and T-tagged (FF-94® from Floy Tag, Seattle, USA). Fish survival after handling and tagging was 99.5 and 95.9% for the Senegal sole and brill, respectively. However, this variable decreased to 73.7% for the wedge sole. It was surely due to the mean size of this species (23.7 ± 0.07 g and 13.4 ± 0.03 cm).
A different identifier code (four digits) and a single free telephone number were printed in every tag. The code was associated with every individual biometric data and the telephone number was which fishermen could call in when captured any tagged fish and provide the next data: fish code, weight and length, fishing gear, date, and location.
Just after every fish release, several informative sessions were carried out along the Gulf of Cadiz coast (from Chipiona, 36° 44′ N; 6° 26′ W, to El Terrón, 37° 13′ N; 7° 10′ W, see Fig. 1). The aim was to collect data about tagged fish in fishermen associations, fish markets, ports, and related. Many flyers and posters were delivered in all these places. The instructions to follow when somebody fished a tagged fish were clearly explained in them.
Tagged fish data were collected from 29 November 2006 to 30 November 30 2008. Several parameters are calculated: individual-specific growth rates (SGR = 100·[ln(final weight) − ln(initial weight)]/time); individual elapsed time (time between releasing and recapture dates); recapture rate (100 number of released fish/number of captured fish); and individual movement (distance between releasing and recapture places).
Natural (M) and fishing (F) mortalities are calculated according to Lin et al. (2010), based on the Gulland (1955) likelihood function:
where N is the number of fish tagged and released, n is the number recaptured, ti is the recapture time for recaptured fish i, m is the combination constant = N!/n!(N–n)!, and T is the last recapture time. F and M were estimated by maximizing L using a non-linear optimization through the Global Optimisation Tool (Generic Algorithm) from MATLAB (MathWorks Inc., MA, USA).
Statistical analyses
Normality and homoscedasticity of all datasets were checked through the Kolmogorov–Smirnov and Levene tests, respectively. Groups were compared through a Student’s t or one-way ANOVA test performing a Duncan post hoc test to detect homogeneous groups (for normal groups), or a Kruskal–Wallis or Mann–Whitney U test (for non-normal groups). Statistical differences amongst capture rate percentages were checked through a Chi-square test. Data are expressed as mean ± standard error of mean. The significance level was 0.05.
Results
Figure 1 shows the map of the South-western Spanish coast, pointing out the artificial reefs and recapture places for all recovered tagged fish. The spatial distribution of fish released in the Matalascañas reef was more disperse than that for the El Rompido reef. In fact, distances were significantly different (p = 0.01): 12.3 ± 10.3 and 6.63 ± 4.45 km (mean ± SD) for Matalascañas and El Rompido reefs, respectively. Moreover, many recaptures took place into and near rivers and river mouths (Guadalquivir, Piedras, and Tinto & Odiel rivers) for all releases. Only seven wedge soles and two brills were recovered, thus their recapture rates were very low, 0.9 and 0.4/0.5% (two different releases), respectively. Therefore, their data could not be processed for the calculation of some post-release parameters.
The summary of post-release data coming from the fishermen information is shown in Table 2. One wedge sole swam the maximum distance, around 58 km (for 65 days) from the release area. However, the highest time in the wild, 159 days, was registered for a Senegal sole. Overall, the wedge soles swam larger distances than Senegal soles, 23.9 ± 8.2 km versus 11.1 ± 0.13 km.
Table 3 shows some post-release parameters depending on initial fish size only for Senegal soles. No significant differences were detected for any parameter amongst sizes (p > 0.5) or releases (p > 0.3). Neither amongst season release, being autumn for ROM and MAT3 and spring for MAT1 and MAT2 (p > 0.3 except for displacement, p = 0.079) nor release site (El Rompido and Matalascañas, p > 0.3). The effects of initial size on recaptures or fish displacements were not significant (p > 0.5). Finally, the release area/reef (El Rompido or Matalascañas) did not affect the final weight or recapture rate, although the initial weight was significantly different between both reefs (Table 4). The relatively high fish size for releasing could have been the reason of the lack of differences between the analysed variables (see Discussion).
The fishing mortality was always higher than the natural one, as shown in Table 5. Moreover, both rates in releases during spring (MAT1 and MAT2) were higher than the others, despite there was no significant difference amongst release parameters (see above, Table 3).
Figure 2 shows the temporal evolution of distance from the release site and time after release. The distance from the release site did not follow a clear pattern over the time, although only after 2 weeks some fish had swum over 15 km. Also, it seems that the most of fish released in Matalascañas reef moved to South (Guadalquivir river mouth). The recapture rates depending on time after release are shown in Fig. 3. Around 80% of recaptures were registered within 15 weeks for every release.
Discussion
These have been the first flatfish stock enhancements carried out in the South of Spain, although other works have been published in Asia, America, and Europe with other flatfish species such as the turbot (Scophthalmus maximus), Japanese flounder (Paralichthys olivaceus), barfin flounder (Verasper moseri), spotted halibut (Verasper variegatus), winter flounder (Pseudopleuronectes americanus Walbaum), and summer flounder (Paralichthys dentatus) (Ishino, 1999; Støttrup et al., 2002; Kellisson et al., 2003; Fairchild et al., 2005; Sparrevohn & Støttrup, 2007; Wada et al., 2012, 2014). Therefore these pioneer stock enhancements in the Spanish Southern coast can provide a first approach on these species biology and restocking efficiency.
Prior to release, fish survival after handling and tagging was 99.5%, 95.9%, and 73.7% for Senegal sole, brill, and wedge sole, respectively. Some researchers have reported that T-tag can decrease the growth in turbot (Støttrup et al., 2002; Paulsen & Støttrup, 2004). In fact, Støttrup et al. (2002) used the colour marking of otoliths with tetracycline or alizarin complexone for smaller fish. Additionally, the external T-tags can cover with mussels and algae, which renders them heavier and more cumbersome, and the relatively sedentary, benthic lifestyle of flatfish may encourage tag fouling, as reported in Støttrup et al. (2002). Nevertheless, the external T-tags can be easily readable for fishermen and their collaboration can be crucial in this type of studies.
The recapture rates obtained in this work for Senegal soles (1.3–5.5%) were similar to those reported in both Perciformes and Pleuronectiformes fish stock enhancements (Bergstad & Folkvord, 1997; Sánchez-Lamadrid, 2002). Although no significant differences were detected amongst groups (Table 3), large and intermediate fish showed higher recapture rates, which leads to hypothesize that survival was better. Similarly, in another flatfish as the turbot, recapture rates (through research surveys) were lower for smaller fish (around 0.16–0.22%), increasing in larger individuals up to 11% (Støttrup et al., 2002).
Otterå et al. (1999) have reported high significant correlations between size and recapture rates in cod (Gadus morhua), and Sánchez-Lamadrid (2004) have shown similar results in gilthead sea bream (Sparus aurata), indicating that 15-g fish registered lower recapture rates due to fish and bird predation and the difficulty to identify small tags. Neither of those hypotheses is applicable to our work because Senegal soles had a significant size, and all tags were identical and clearly visible. In that sense, it has been established that the dependence between size and recapture may be higher for roundfish species with high vulnerability to predation and cannibalism than for flatfish species (Støttrup et al., 2002). Anyway, it is logical to suppose that bigger fish have more probabilities to be fished by typical fishing gears (gillnets and related) than smaller ones.
In other flatfish species such as Pseudopleuronectes americanus, low cryptic abilities have related to low released fish survival (Fairchild & Howell, 2004). Although there is no evidence on the cryptic changes in released Senegal soles, it is expectable that this species would adapt its colour skin quickly, as several works have been demonstrated in laboratory experiments (Ruane et al., 2004; Rodiles et al., 2005).
Analysing the recapture rates evolution (Fig. 3), it seems that around 80% of recaptures were registered within the first 15-week post-release, reaching the total rate at 23 week. Sánchez-Lamadrid (2004) reported tagged gilthead sea breams (Sparus aurata) caught within 50–100-week post-release, the most of them (80–90%) being recaptured during the first 28–43 weeks. Those differences could be due to the final total amount of tagged fish, which was much higher than ours.
The release site and its hydrodynamic and ecological features can affect the fish survival, especially in semi-closed areas. However, our releases were performed in open sea artificial reefs and, in fact, no difference for any parameter was detected amongst release sites (El Rompido and Matalascañas). The estimation of survival through recapture rates is difficult in so large areas, and recapture rates usually are lower. Contrarily, Nakagawa et al. (2007) and Wada et al. (2012) have reported recapture rates over 15% in Japanese bays.
According to Sánchez-Lamadrid (2004), a high recapture rate is related to food abundance. We did not find significant differences for any parameter amongst release site or date although MAT1 showed the highest recapture rate. The fishing pressure is another factor involving in the recapture rates, nevertheless that parameter is relatively stable in the Andalusian coast all-year-around except for July and August, when it grows and, paradoxically, no recapture was registered. Therefore, it is difficult to explain the higher recapture rate in the MAT1 release, when fishing pressure was lower than that for MAT2. Perhaps the bigger fish released and the high natural spring productivity which would improve the fish survival and growth, could justify that higher recapture rate. Nevertheless, as said before, the differences were not significant amongst releases.
No significant differences for distance were found amongst sizes. The release site also can affect the fish movements. According to Fairchild et al. (2005), generally, the more favourable the release site, the less dispersion there is. In fact, tagged turbots released in Galicia (Spanish North-western coast) swam up to 9 km when were released in a 25-m depth area; this distance significantly grew when the depth was higher than 25 m (Iglesias & Rodríguez-Ojea, 1994). Nevertheless, the results in Norway were different since a fish dispersion within a 50 km radius or higher (Bergstad & Folkvord, 1997). In our work, the maximum displacement was registered for a wedge sole (58 km), and it could be related to the swimming behaviour of this species, being more active than other flatfish (Herrera et al., 2008). The maximum displacement for the Senegal sole was 55 km, belonging to a fish released in the Matalascañas reef.
Moreover, fish dispersion in El Rompido release was significantly lower than those in Matalascañas reef (see Fig. 1 and Results). It has been reported that river mouths and estuaries are high productivity areas (Broadley et al., 2022). In fact, the Guadalquivir mouth and adjacent marine waters have been described as an important nursery ground for several commercial species in the Gulf of Cadiz (Llope, 2016). The difference between fish dispersion in our work seems to be related to the fish movements to look for higher productivity areas. Therefore, fish released in El Rompido did not displace meaningfully since they were close to the Piedras and Tinto & Odiel river mouths; however, fish from the Matalascañas reef swam to the Guadalquivir mouth, supposing a higher displacement.
The effects of these flatfish releases on the fishery production are very difficult to assess. The low amount of fish released and short time of these activities are the major factors. According to Fig. 4, Senegal sole catches in the Gulf of Cadiz grew from the first stock enhancements although we cannot evidence a relationship amongst commercial captures and releases. At least, it seems clear that the releases did not affect the fishery production negatively. The establishment of long-term stock enhancements programmes and projects could be a useful tool to evaluate the effects on the recovery of natural stocks.
Concluding, after the first hatchery-produced flatfish releases in the Gulf of Cadiz, we can conclude that the design of long-term stock enhancement programmes in the Gulf of Cadiz according to the described methodology is feasible and necessary to recover commercial and endangered species stocks. Future programmes should also include research surveys besides the fishermen information to achieve more data and accurate information. Additionally, laboratory experiments on fish tagging and its effects on fish survival, growth, and welfare are necessary.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Andersen, A. K., J. Schou, C. R. Sparrevohn, H. Nicolajsen & J. G. Støttrup, 2005. The quality of a release habitat for reared flounder, Platichthys flesus, with respect to salinity and depth. Fisheries Management and Ecology 12: 211–219.
Bauchot, M.L., 1987. Soleidae. In Fischer, W., M.L. Bauchot & M. Schneider (eds), FAO Fish Identification Guide for Fishery Purposes (Revision 1). Mediterranean and Black seas. Fishery zone 38, Vol. II. Vertebrates. FAO, Rome: 761–763.
Belghyti, D., O. Berrada-Rkhami, V. Boy, P. Aguesse & C. Gabrion, 1994. Population biology of two helminth parasites of flatfishes from the Atlantic coast of Morocco. Journal of Fish Biology 44: 1005–1021.
Bell, J. D., D. M. Bartley, K. Lorenzen & N. R. Loneragan, 2006. Restocking and stock enhancement of coastal fisheries: potential, problems and progress. Fisheries Research 80: 1–8.
Ben-Tuvia, A., 1990. A taxonomic reappraisal of the Atlanto-Mediterranean soles Solea solea, Solea senegalensis and Solea lascaris. Journal of Fish Biology 36: 947–960.
Berejikian, B. A., D. A. Larsen, P. Swanson, M. E. Moore, C. P. Tatara, W. L. Gale & B. R. Beckman, 2012. Development of natural growth regimes for hatchery-reared steelhead to reduce residualism, fitness loss, and negative ecological interactions. Environmental Biology of Fishes 94: 29–44.
Bergstad, O. A. & A. Folkvord, 1997. Dispersal of tagged juvenile turbot Scophthalmus maximus on the Norwegian Skagerrak coast. Fisheries Research 29: 211–215.
Bolstad, G. H., K. Hindar, G. Robertsen, B. Jonsson, H. Sægrov, O. H. Diserud & B. T. Barlaup, 2017. Gene flow from domesticated escapes alters the life history of wild Atlantic salmon. Nature Ecology & Evolution 1: 0124.
Broadley, A., B. Stewart-Koster, M. A. Burford & C. J. Brown, 2022. A global review of the critical link between river flows and productivity in marine fisheries. Reviews in Fish Biology and Fisheries. https://doi.org/10.1007/s11160-022-09711-0.
Cabral, H. N., M. Lopes & R. Loeper, 2002. Trophic niche overlap between flatfishes in a nursery area on the Portuguese coast. Scientia Marina 66(3): 293–300.
Chen, P., C. Qin, J. Yu, L. Shu, X. Li, Y. Zhou & H. Yuan, 2015. Evaluation of the effect of stock enhancement in the coastal waters of Guangdong, China. Fisheries Management and Ecology 22: 172–180.
Christie, M. R., M. L. Marine, R. A. French & M. S. Blouin, 2012. Genetic adaptation to captivity can occur in a single generation. Proceedings of the National Academy Science 109: 238–242.
Consejería de Agricultura y Pesca, 2001. Especies de Interés Pesquero en el Litoral de Andalucía, Junta de Andalucía, Sevilla:
Consejería de Agricultura, Pesca y Desarrollo Rural, 2017. Junta de Andalucía (Regional Government) Estadísticas Pesqueras. http://www.juntadeandalucia.es/organismos/agriculturapescaydesarrollorural/consejeria/sobre-consejeria/estadisticas/paginas/pesqueras-pub.html
Desoutter, M., 1990. Soleidae. In J.C. Quero, J.C. Hureau, C. Karrer, A. Post and L. Saldanha (eds) Check-list of the Fishes of the Eastern Tropical Atlantic (CLOFETA). Vol. 2. JNICT, Lisbon; SEI, Paris; and UNESCO, Paris: 1037–1049.
Dinis, M. T., 1986. Quatre soleidae de L’Estuaire du Tage: Reproduction et Croissance. Essai d’elevage de Solea senegalensis Kaup, Université de Bretagne Occidentale, France:
Fairchild, E. A. & W. H. Howell, 2004. Factors affecting the post-release survival of cultured juvenile Pseudopleuronectes americanus. Journal of Fish Biology 65: 69–87.
Fairchild, E. A., J. Fleck & W. H. Howell, 2005. Determining an optimal release site for juvenile winter flounder Pseudopleuronectes americanus (Walbaum) in the Great Bay Estuary, NH, USA. Aquaculture Research 36: 1374–1383.
FAO, 2018. FishStat Plus Universal Software for Fishery Statistical Time Series, FAO Fisheries and Aquaculture Department, Rome:
Garcia-Franquesa, E., A. Molinero, J. Valero & R. Flos, 1996. Influence of sex, age and season on the feeding habits of the flatfish Solea senegalensis. Environmental Biology of Fishes 47: 289.
Gonzalez, E. B., M. Aritaki, H. Knutsen & N. Taniguchi, 2015. Effects of large-scale releases on the genetic structure of red sea bream (Pagrus major, Temminck et Schlegel) populations in Japan. PLoS One 10(5): e0125743.
Grant, W. S., J. Jasper, D. Bekkevold & M. Milo Adkison, 2017. Responsible genetic approach to stock restoration, sea ranching and stock enhancement of marine fishes and invertebrates. Reviews in Fish Biology and Fisheries 27: 615–649.
Gulland, J. A., 1955. On the estimation of population parameters from marked members. Biometrika 42: 269–270.
Hachero-Cruzado, I., J. B. Ortiz-Delgado, B. Borrega, M. Herrera, J. I. Navas & C. Sarasquete, 2009. Larval organogenesis of flatfish brill Scophthalmus rhombus L: histological and histochemical aspects. Aquaculture 286: 138–149.
Hagen, I. J., U. Ola, A. J. Jensen, L. Håvard, E. Holthe, B. Bjøru, B. Florø-Larsen, H. Sægrov, H. Skoglund & S. Karlsson, 2020. Evaluation of genetic effects on wild salmon populations from stock enhancement. ICES Journal of Marine Science 78: 900–909.
Herrera, M., I. Hachero, M. Rosano, J. F. Ferrer, J. M. Márquez & J. I. Navas, 2008. First results on spawning, larval rearing and growth of the wedge sole (Dicologoglossa cuneata) in captivity, a candidate species for aquaculture. Aquaculture International 16: 69–84.
Iglesias, J. & G. Rodriguez-Ojea, 1994. Fitness of hatchery-reared turbot, Scophtha1mus maximus L., for survival in the sea: first year results on feeding, growth and distribution. Aquaculture and Fisheries Management 25: 179–188.
Ishino, K., 1999. Stocking effectiveness of Japanese flounder, Paralichthys olivaceus, fingerlings released on the south-western coast of Hokkaido Prefecture, Japan. In Howell, B. R., E. Moksness & T. Svåsand (eds), Stock Enhancement and Sea Ranching Blackwell Publishing, Oxford: 334–339.
Jiménez, M. P., I. Sobrino & F. Ramos, 1998. Distribution pattern, reproductive biology and fishery of the wedge sole Dicologoglossa cuneata in the Gulf of Cádiz, south-west Spain. Marine Biology 131: 173–187.
Kellison, G. T., D. B. Eggleston, J. C. Taylor, J. S. Burke & J. A. Osborne, 2003. Pilot evaluation of summer flounder stock enhancement potential using experimental ecology. Marine Ecology Progress Series 250: 263–278.
Kitada, S., 2018. Economic, ecological and genetic impacts of marine stock enhancement and sea ranching: a systematic review. Fish and Fisheries 19: 511–532.
Kitada, S. & H. Kishino, 2006. Lessons learned from Japanese marine finfish stock enhancement programmes. Fisheries Research 80: 101–112.
Kitada, S., Y. Taga & H. Kishino, 1992. Effectiveness of a stock enhancement program evaluated by a two-stage sampling survey of commercial landings. Canadian Journal of Fisheries and Aquatic Sciences 49: 1573–1582.
Leber, K. M. & S. M. Arce, 1996. Stock enhancement in a commercial mullet, Mugil cephalus, L., fishery in Hawaii. Fisheries Management 3: 261–278.
Lin, Y. J., S. L. Chang, M. Y. Chang, S. H. Lin, T. I. Chen, M. S. Su, W. C. Su & W. N. Tzeng, 2010. Comparison of recapture rates and estimates of fishing and natural mortality rates of Japanese Eel Anguilla japonica between different origins and marking methods in a mark-recapture experiment in the Kaoping river, Southern Taiwan. Zoological Studies 49(5): 616–624.
Llope, M., 2016. The ecosystem approach in the Gulf of Cadiz. A perspective from the southernmost European Atlantic regional sea. ICES Journal of Marine Science 74: 382–390.
Nakagawa, M., H. Okouchi, J. Adachi, K. Hattori & Y. Yamashita, 2007. Effectiveness of stock enhancement of hatchery-released black rockfish Sebastes schlegeli in Yamada Bay – evaluation by a fish market survey. Aquaculture 263: 295–302.
Nakajima, K., S. Kitada, H. Yamazaki, H. Takemori, Y. Obata, A. Iwamoto & K. Hamasaki, 2013. Ecological interactions between hatchery and wild fish: a case study based on the highly piscivorous Japanese Spanish mackerel. Aquaculture Environment Interactions 3: 231–243.
Otterå, H., T. S. Kristiansen, T. Svåsand, M. Nødtvedt & A. Borge, 1999. Sea ranching of Atlantic cod (Gadus morhua L.): effects of release strategy on survival. In Howell, B. R., E. Moksness & T. Svåsand (eds), First International Symposium on Stock Enhancement and Sea Ranching Fishing News Books/Blackwell Scientific Publications, Oxford: 293–305.
Paulsen, H. & J. G. Støttrup, 2004. Growth rate and nutritional status of wild and released reared juvenile turbot in southern Kattegat, Denmark. Journal of Fish Biology 65(Suppl. A): 210–230.
Pérez, G., 2004. C. In Rey, M., J. Fernández, M. Izquierdo & A. Guerra (eds), VII Foro dos Recursos Mariños e da Acuicultura das Rias Galegas Asociación Cultural do Foro dos Recursos Mariños e a Acuicultura das Rías Galegas, O Grove: 51–58.
Pérez, M. & F. Rodríguez, 2001. Especies de interés pesquero en el litoral de Andalucía., Vertebrados Junta de Andalucía, Consejería de Agricultura y Pesca, Sevilla: 388.
Ramos, F., I. Sobrino & M. P. Jiménez, 1996. Cartografía temática de caladeros de la flota de arrastre en el Golfo de Cádiz, Servicio de Publicaciones y Divulgación:, 1–44.
Ribeiro, L., J. L. Zambonino-Infante, C. Cahu & M. T. Dinis, 1999. Development of digestive enzymes in larvae of Solea senegalensis, Kaup 1858 L.). Aquaculture 179: 465–473.
Rodiles, A., M. Herrera, I. Hachero, J. F. Ferrer, M. Rosano, J. M. Márquez & J. I. Navas, 2005. Influence of different bottom types on the Senegal sole Solea senegalensis Kaup, 1858 ongrowing. Boletin Del Instituto Español De Oceanografía 21(1–4): 195–199.
Ruane, N. M., F. Soares & M. T. Dinis, 2004. Background adaptation in Solea senegalensis: not just black andwhite. European Aquaculture Society Special Publication 34: 698–699.
Rutledge, W. P., 1989. The Texas marine hatchery program – it works! California Cooperative Oceanic Fisheries Investigations 30: 49–52.
Sánchez-Lamadrid, A., 2002. Stock enhancement of gilthead sea bream (Sparus aurata L.): assessment of season, fish size and place of release in SW Spanish coast. Aquaculture 210: 187–202.
Sánchez-Lamadrid, A., 2004. Effectiveness of releasing gilthead sea bream (Sparus aurata, L.) for stock enhancement in the bay of Cádiz. Aquaculture 231: 135–148.
Sparrevohn, C. R. & J. G. Støttrup, 2007. Post-release survival and feeding in reared turbot. Journal of Sea Research 57: 151–161.
Støttrup, J. G. & C. R. Sparrevohn, 2007. Can stock enhancement enhance stocks? Journal of Sea Research 57: 104–113.
Støttrup, J. G., C. R. Sparrevohn, J. Modin & K. Lehmann, 2002. The use of releases of reared fish to enhance natural populations. A case study on turbot Psetta maxima (Linné, 1758). Fisheries Research 59: 161–180.
Svåsand, T., T. S. Kristiansen, T. Pedersen, A. G. V. Salvanes, R. Engelsen & G. Nævdal, 2000. The enhancement of cod stocks. Fish and Fisheries 1: 173–205.
Szuwalski, C. S., K. A. Vert-Pre, A. E. Punt, T. A. Branch & R. Hilborn, 2015. Examining common assumptions about recruitment: a meta-analysis of recruitment dynamics for worldwide marine fisheries. Fish and Fisheries 16: 633–648.
Wada, T., K. Kamiyama, S. Shimamura, T. Mizuno & Y. Nemoto, 2012. Effectiveness of stock enhancement of a rare species, spotted halibut Verasper variegatus, in Fukushima, Japan. Aquaculture 364: 230–239.
Wada, T., K. Kamiyama, S. Shimamura, O. Murakami, T. Misaka, M. Sasaki & T. Kayaba, 2014. Fishery characteristics of barfin flounder Verasper moseri in southern Tohoku, the major spawning ground, after the start of large-scale stock enhancement in Hokkaido, Japan. Fisheries Science 80: 1169.
Walsh, M., 2013. Conditioning technologies for flatfish stock enhancement: global progress and pitfalls, NOAA Technical Memorandum NMFS-F/SPO-136: 44–50.
Yamashita, Y. & H. Yamada, 1999. Release strategy for Japanese flounder fry in stock enhancement programmes. In Howell, B. R., E. Moksness & T. Svåsand (eds), First Int Symp. Stock Enhancement and Sea Ranching. Fishing News Books Blackwell Scientific Publications, Oxford: 191–204.
Yang, K., S. Li, X. Liu, W. Gan, L. Deng, Y. Tang & Z. Song, 2017. Mass marking of juvenile Schizothorax wangchiachii (Fang) with alizarin red S and evaluation of stock enhancement in the Jinping area of the Yalong River. PeerJ 5: e4142.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study has been financed by the Consejería de Agricultura, Ganadería, Pesca y Desarrollo Sostenible of the Junta de Andalucia. M. Herrera’s post-doc contract is supported by the National Institute of Agricultural Research and the European Social Fund (INIA-FSE). Funding for open access charge: Universidad de Huelva / CBUA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Handling editor: Grazia Pennino
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Herrera, M., Rodiles, A., Salamanca, N. et al. First releases of hatchery-produced Senegal sole (Solea senegalensis), brill (Scophthalmus rhombus), and wedge sole (Dicologoglossa cuneata) juveniles in the South-western Spanish coast. Hydrobiologia 850, 203–214 (2023). https://doi.org/10.1007/s10750-022-05054-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05054-w