Abstract
The concept of native range in invasion biology is difficult to define since, in many cases, this type of range is unknown and cannot be determined. We investigate the uncertainties related to this concept by focusing on the distribution of Faxonius rusticus (Girard, 1852), also known as the rusty crayfish, which is perceived as possibly the worst invasive crayfish species in North America. In this study, we undertake a comprehensive literature review, which includes 430 studies published between 1852 and 2018, in order to analyze the native and introduced ranges of this species. The rusty crayfish was reported to occur in 33 states in the U.S.A. and 3 Canadian Provinces. Ten of these U.S. states and one Canadian Province have been included multiple times in both the native and the non-native ranges of this crayfish. The confusion regarding the limits and history of the native range of the rusty crayfish has implications for the conservation of this species in various jurisdictions. This review also demonstrates that even for intensely studied species perceived as invasive, we often do not have a clear understanding of essential concepts such as native and non-native range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The geographic distributions of species have been a major topic of interest for naturalists and biologists for centuries, as shown, for example, in the classic works of Alexander von Humboldt (1805) and Alfred Russel Wallace (1876). Charles Darwin dedicated two chapters of his seminal book On the origin of species (1859) to the geographical distribution of species, and mentioned interesting examples of long-distance dispersal, such as the transport of plant seeds and freshwater molluscs on the muddy feet of birds. Almost one century later, British ecologist Charles Elton published the book The ecology of invasions by animals and plants (1958), which is generally acknowledged as the starting point of the young field of invasion biology. The rise of invasion biology, however, did not start in earnest until decades later, in the early 1990s (Ricciardi & MacIsaac, 2008; Guiaşu, 2016; Guiaşu & Tindale, 2018).
As invasion biology started growing rapidly and becoming more influential in the last three decades or so, researchers in this field began to focus more and more on concepts such as native range, in order to determine the native or non-native status for an ever-expanding number of species (Guiaşu, 2016). Despite this increased focus, leading invasion biologists agree that the concept of native range still lacks a formal theoretical definition in invasion biology (Courchamp et al., 2020; Pereyra, 2020). Identifying non-native species is based on the assumption that the native range is known or can be determined. Nevertheless, this is not the case in many instances (Guiaşu, 2016; Orlova-Bienkowskaja & Volkovitsh, 2018; Pereyra, 2020). Thus, the task of differentiating native species from non-native species can prove to be rather difficult, or virtually impossible, in many cases since the limits and history of native ranges, as well as the diverse means of species’ dispersal, are often unknown (Guiaşu, 2016; Essl et al., 2018; Guiaşu & Tindale, 2018; Pereyra & Guiaşu, 2020).
In his book about the dynamic nature of the distributions of species, Gaston (2003) stated that the study of the limits of the geographic ranges of species “should be a central objective of ecological research,” before discussing some of the challenges associated with the determination of such limits and the current general lack of a “comprehensive understanding of why any given species occurs where it does and not elsewhere.” The subjective nature of the native range concept was discussed in detail by Guiaşu (2016) and Guiaşu & Tindale (2018). Even more recently, a rather intense debate about the definition and usefulness of the native range concept in invasion biology was featured in the pages of the journal Conservation Biology (Courchamp et al., 2020; Pereyra, 2020; Pereyra & Guiaşu, 2020). Pereyra & Guiaşu (2020) argued that the concept of native range is not well defined, not universal, and therefore not useful in quite a few cases in conservation biology, and in invasion biology in particular.
Determining the limits and history of native ranges can be particularly challenging in situations where species expand their ranges by moving into adjacent areas which are not separated from the assumed previous ranges by any insurmountable geographic barriers. In such cases, we often have no way of knowing if the species expanded their ranges entirely on their own, without human assistance, or with the direct or indirect help of people. One such example is provided by Faxonius rusticus (Girard, 1852), also known as the rusty crayfish, in North America. This freshwater species has been expanding its North American range in the last several decades often by moving through interconnected rivers and lake systems (Guiaşu, 2016; Guiaşu & Tindale, 2018).
Even though the rusty crayfish is indeed unquestionably native to North America, this species is currently considered invasive in many regions of this continent, and the appearance of this crayfish at new locations is generally treated as an “invasion,” despite the frequent absence of sufficient historical biogeographic data and persuasive information about the means of dispersal (Guiaşu, 2016). As a result of the “invasive” label often rather casually applied to this species, the rusty crayfish is the target of control programs that seek to eradicate it from certain lakes and streams (Hein et al., 2006, 2007). However, the exact extent of the native range and the means of dispersal of this crayfish species remain unclear at least in some regions (Guiaşu, 2016).
In the current study, we analyze the distribution of the rusty crayfish (Faxonius rusticus) in North America by reviewing all the information we could find on this topic in the scientific literature, and discuss the uncertainties about the extent of the native and non-native ranges of this species. Our aim is to use this well-known and much studied species as a cautionary example of the potential problems with the native range concept.
Materials and methods
Literature search
In order to review the literature on the rusty crayfish, the following terms were used for a search on Google Scholar (last accessed on March 19, 2019): rusty crayfish; Orconectes rusticus (a previous name for this species used in the majority of the references cited in this study); Faxonius rusticus (a newer name assigned to the species in 2017); and Cambarus rusticus (the older name initially assigned to this species by Girard in 1852, before the change to the genus Orconectes). This comprehensive literature search yielded 430 publications, consisting mainly of peer-reviewed journal articles, as well as a few scientific books, technical reports, and academic dissertations. Of these publications, 412 were accessed online through a York University subscription with Google Scholar, while The Crayfishes of Ohio (Turner, 1926) and the Ecology of the Crayfish Orconectes rusticus in Northern Wisconsin (Lorman, 1980) works were acquired physically through an interlibrary loan with Lakehead University. Sixteen other publications were accessed from Radu Guiaşu’s personal reprint and book collection (Huntsman, 1911; Hobbs, 1974; Smith, 1981; Hobbs & Jass, 1988; Maude, 1988; Momot et al., 1988; Jezerinac et al., 1995; Momot, 1996; Hamr, 1998; Holdich, 2002; Lieb et al., 2007; Hamr, 2010; Nadelhoffer et al., 2010; Rosenburg et al., 2010; Somers & Reid, 2010; Booy et al., 2015). Thirty-one other publications could not be accessed at all, either electronically or physically. These 31 publications were not included in the analysis. Given the extensive nature of the literature search, and the fact that we were able to access and analyze the information in 430 specialized publications, published between 1852 and 2018, we do not believe that the absence of the 31 studies we could not access would have a significant impact on the conclusions drawn from the examination of our vast data set.
Native and introduced range survey
In order to gather the information on the native or non-native range assignments for the rusty crayfish in various jurisdictions, and identify the potential discrepancies with regard to the native and introduced ranges for this species, all 430 accessible publications were read very carefully, and the relevant information was tabulated (Table 1 and Online Appendix A). Only clear and specific references to particular U.S. states or Canadian Provinces were included in the data set shown in Table 1. More ambiguous references, such as “Ohio River basin” or “Ohio River valley” were not included, because the Ohio River basin covers 14 states in the U.S.A., and therefore we found that such descriptions were too vague to allow us to assign the distribution to any particular state or states. On the other hand, reports such as “Ohio River at Cincinnati” were included among the data in Table 1, since the Cincinnati location is clearly found within the state of Ohio.
Information about the F. rusticus distribution was classified into three separate categories. The first two categories were composed of clearly defined native and introduced ranges, respectively, given in studies where authors explicitly mentioned the native and/or non-native status of the rusty crayfish in well-defined jurisdictions (U.S. states and Canadian Provinces). A third category was used in cases where rusty crayfish geographical data were presented without any specification as to whether the described regions were part of the species’ native range or introduced range. When the range in a particular category was not discussed at all in a study, this was indicated in the Appendix A table as well. This allowed us to understand the frequency with which various types of ranges were discussed in the scientific literature which focused, exclusively or partially, on the rusty crayfish. The 430 studies listed in Online Appendix A are arranged in chronological order, beginning in 1852 and ending in 2018.
For the purposes of our analysis, we have also divided the data set into two unequal time periods: 1) the period between 1852 (the year of the first study we found which mentioned this crayfish species) and 1989 (a period of 137 years); and 2) the 28-year-long period between 1990 (starting with the Bruski & Dunham, 1990 study) and 2018 (the last year included in our data set). While any such subdivision of the data set can be somewhat arbitrary, we chose the year 1990 as the first year of the second time period in our analysis, because there has been a dramatic rise in the number of publications in the young field of invasion biology since the early 1990s (Ricciardi & MacIsaac, 2008; Guiaşu, 2016; Guiaşu & Tindale, 2018). Since the rusty crayfish is considered to be an invasive species, we wanted to find out if there was an increased interest in this species, and its native or non-native ranges in particular, starting in the 1990s, as a result of the rise of invasion biology.
In general, crayfish behavioral and physiological studies, for example many of those from the labs of researchers such as David Dunham, Brian Hazlett, Robert Huber, Paul Moore, and Ann Jane Tierney, did not discuss species distributions and were not concerned with biogeographic concepts such as native or non-native ranges. However, when such studies involved the collection of rusty crayfish in a well-defined jurisdiction (such as Ohio, Michigan, or Ontario, for example), we included this information in our data set, usually in the unspecified range category (unless the native or non-native status of the rusty crayfish was clearly mentioned).
Recently, Crandall & De Grave (2017) assigned the surface water crayfish previously grouped in the genus Orconectes Cope 1872, including Orconectes rusticus, to the genus Faxonius Ortmann, 1905. Thus, all the Faxonius species mentioned in this study used to be placed in the genus Orconectes prior to the publication of the Crandall & De Grave (2017) study. We follow this updated classification, and use the new Faxonius rusticus (Girard, 1852) scientific species name for the rusty crayfish in this study.
Results
The comprehensive literature review included 430 studies published between 1852 and 2018—a span of 166 years (Online Appendix A).
We compared the two time periods—the one between 1852 and 1989 and the one between 1990 and 2018—to determine the possible effect of invasion biology on the number of studies published. Overall, 139 studies mentioning the rusty crayfish were published between 1852 and 1989. Thus, during this period, which covers 137 years, an average of 1.01 studies per year which referred to the rusty crayfish were published. However, during the subsequent 28 years, between 1990 and 2018, 291 studies mentioning the rusty crayfish were published, and, therefore, an average of 10.39 such studies per year were published during this much shorter period. Thus, the number of studies published per year increased more than tenfold between 1990 and 2018, in comparison to the period covering the previous 137 years.
When we analyzed the numbers of times, particular range types (native, non-native, or unspecified) were not discussed in the studies included in our data set (Appendix A), we obtained the following numbers: 742 “not discussed” entries for the entire data set (1852–2018); 268 (or 36.12% of the total) “not discussed” entries for the 1852–1989 period; and 474 (or 63.88% of the total) “not discussed” entries for the 1990–2018 period.
Looking at the data in more detail, we found that the 268 “not discussed” entries for the 1852–1989 period were subdivided in the following way: native range was not discussed in 113 studies, non-native range was not discussed in 97 studies, and the unspecified range (range not clearly identified as either native or non-native) was not discussed in 58 studies. During the 1852–1989 period, all three types of ranges were not discussed at all in only 18 (12.95%) of the 139 studies. So, less than 13% of the studies published during this period failed to mention the geographic range of the rusty crayfish altogether. During this same period (1852–1989), neither native nor non-native ranges were mentioned in 97 studies (or 69.78% of the total number of 139 studies for this period). If we subtract the number of studies (18) where range was not mentioned at all, we are left with 79 studies (or 56.83% of the total) in which range was mentioned but not identified as either native or non-native. This means that in more than one half of the studies published during this 137-year period, no distinction was made between native and non-native ranges. The data also show that between 1852 and 1989, the non-native range was mentioned in 16 more studies than the native range. On the other hand, there were no studies published between 1852 and 1989 in which the native range was mentioned without the non-native range. So, the native range was never mentioned alone in any of these studies, without the non-native range being discussed as well.
The 474 “not discussed” range entries recorded during the 1990–2018 period were subdivided in the following way: native range was not discussed in 158 studies; non-native range was not discussed in 104 studies; and the unspecified range (native or non-native range not clearly identified) was not discussed in 212 studies.
During the 1990–2018 period, none of the three types of ranges were discussed at all in 25 (or 8.59%) of the 291 studies published. During this same period, neither native nor non-native ranges were mentioned together in 97 (or 33.33%) of the total 291 studies published. If we subtract the 25 studies during which the range was not mentioned at all, we are left with 72 studies (or 24.74% of the total) in which range was mentioned but not identified as either native or non-native. So, in about one-quarter of the studies published between 1990 and 2018, the range was mentioned, but no distinction was made between native and non-native ranges. Clearly, the percentage of studies in which range was mentioned but not clearly identified as either native or non-native decreased substantially over time, from almost 57% during the 1852–1989 period to less than 25% between 1990 and 2018. Thus, after 1990, a much higher percentage of studies than before identified range as either native or non-native.
During the 1990–2018 period, the non-native range was mentioned alone (without the native range also being mentioned) in 61 studies, whereas the native range was mentioned alone (without the non-native range also being mentioned) in only 7 studies. Therefore, between 1990 and 2018, non-native range was mentioned without the native range in almost nine times more studies than the other way around. This suggests a predominant focus on the non-native, or introduced, range of the rusty crayfish in quite a few studies.
For the entire period included in our study (1852–2018), non-native range was mentioned alone (without the native range being also mentioned) in 77 studies, whereas the native range was mentioned alone (without the non-native range also being mentioned) in only 7 studies.
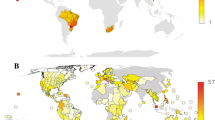
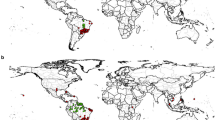
The data showing the numbers of states in the U.S.A. and Canadian Provinces where F. rusticus was listed as either native, or non-native, or both, are shown in Table 1. According to the data summarized in Table 1, F. rusticus was reported at least once from 33 states in the U.S.A. and 3 Canadian Provinces. Ten states in the U.S.A. and one Canadian Province (Ontario), representing about one third of the United States and Canadian jurisdictions included in the analysis, respectively, had both native and non-native listings reported for the rusty crayfish. Of the 20 U.S. states where only one range type (native or non-native) was listed, 2 were listed only in the native range category, 2 were listed in the native range and unspecified range-type categories, 11 were listed only in the non-native (or introduced) range category, and 5 were listed in the non-native range and unspecified range-type categories. The remaining 3 U.S. states were listed only in the unspecified (native or non-native status unclear) category (Table 1). The two Canadian Provinces (Manitoba and Quebec) for which only one type of range was given only had non-native range listings for F. rusticus.
Discussion
Faxonius rusticus currently has a wide range in North America and the presence of this species was reported in 33 U.S. states and 3 Canadian Provinces (Table 1). Of course, as one of us (Guiaşu, 2016) has pointed out before, the borders of states and provinces are simply lines on a map, drawn for political reasons, which have no significance for any other species than our own. These artificial borders generally have no ecological meaning and most often do not separate distinct ecosystems. Nevertheless, since the vast majority of the studies we analyzed refer to the distribution of crayfish in various states and provinces, and since these regions are clearly defined on maps, we are also using U.S. states and Canadian Provinces as indicators of where the rusty crayfish may be found and how the range of this species may have changed over time.
The prevalent current expert opinion seems to be that the rusty crayfish is native to the Ohio River basin and the midwestern areas of the U.S.A., although the exact history, extent, and limits of this native range remain unclear (Dresser & Swanson, 2013; Guiaşu, 2016). There are many disagreements in the scientific literature about some of the regions which may, or may not, be part of the native range of this crayfish species. These disagreements have conservation implications. Most of these disagreements focus on the 10 U.S. states (Ohio, Wisconsin, Michigan, Illinois, Tennessee, Iowa, Minnesota, Missouri, West Virginia, and Virginia) and the one Canadian Province (Ontario) which have been included, in various studies, in the native range and the non-native range of F. rusticus. In certain studies, some of these states were included in both of those ranges at the same time (for example: Taylor, 2000). Among these 10 U.S. states, there are only 4 (Ohio, Michigan, Illinois, and Tennessee) for which we have double digit numbers of entries in both the native range and the non-native range columns (Table 1). Therefore, we would expect most of the controversies and disagreements regarding the type of range (native or non-native) assigned to the rusty crayfish to be related to these particular 4 states, and, as a result, we will analyze the information regarding these 4 states first and in more detail.
Ohio
As can be seen in Table 1, Ohio was listed as part of the native range of the rusty crayfish much more often than in the non-native range category (62 to 11 studies, respectively), although a majority of studies (96) mentioning Ohio, including the first 19 listings for this state in Appendix A, did not specify whether F. rusticus was native or non-native there. Rhoades (1944a) was the first to include Ohio specifically into the native range of the rusty crayfish. The second study which assigned Ohio to the native range of this species was the one by Crocker & Barr (1968). Although Rhoades (1944a) also seemed to place Ohio within the non-native range of F. rusticus, this information was somewhat ambiguous. The first unambiguous inclusion of Ohio into the non-native range of the rusty crayfish was found in a study by Jezerinac (1982), which was published 130 years after the work by Girard (1852) where some information about the distribution of this species was first mentioned. More confusingly, perhaps, in some studies, certain regions of Ohio were declared as being part of the native range of the rusty crayfish, while other regions of this state were considered parts of the non-native range. These regional range assignments were sometimes different in various studies. For example, Butler & Stein (1985) regarded F. rusticus as native in western Ohio, but invasive in east-central and southern Ohio. Rahel (1988) and Jezerinac (1991) considered eastern Ohio as part of the non-native range of F. rusticus. However, Tierney & Dunham (1984) declared the rusty crayfish native to eastern Ohio, and Lorman (1975) thought that this crayfish species was native to northeastern Ohio. The southwestern, or southwest, Ohio region was listed specifically as part of the F. rusticus range in 9 studies. Seven of these studies (Perry et al., 2001, 2002a, b; Dubé & Desroches, 2007; Davis et al., 2012; Roth et al., 2012; and Peters & Lodge, 2013) included southwestern Ohio in the native range of the rusty crayfish, but St. John (1991) disagreed, and assigned this same part of Ohio to the non-native range, while Claussen et al. (2000) did not specify whether southwestern Ohio belonged to the native or the non-native range.
Michigan
In this state, the rusty crayfish is listed as native in 24 studies and non-native in 27 studies, while the type of range (native or non-native) is not specified in 19 studies. The first 9 listings referring to Michigan in Appendix A do not specify the type of range (native or non-native) this state belongs to. Crocker & Barr (1968) were the first to assign Michigan to a specific type of range, and these authors found the rusty crayfish to be native in this state. The study by Charlebois (1994) was the first to include Michigan in the non-native range of F. rusticus, and Momot (1996) and Lodge et al. (1998) followed suit. In fact, after the mid-1990s, it seems that including Michigan in the non-native range for this crayfish species became the dominant position, and this coincided with the rise of invasion biology. Taylor (2000) found that the rusty crayfish was native to southeastern Michigan but non-native in the rest of that state. Three other studies (Lorman, 1975; Capelli, 1982; and Capelli & Magnuson, 1983) specifically found the rusty crayfish to be native to southern Michigan, while DiStefano et al. (2009) were even more specific, and thought that F. rusticus was native to the “southeastern corner of Michigan.” On the other hand, Keller & Hazlett (2010) found that the rusty crayfish was non-native to “northern and lower Michigan.”
Illinois
This is another state which has been included several times in both the native and the non-native range categories for the rusty crayfish—15 times in the native range column and 23 times in the non-native range column, respectively, as well as 13 times in the unspecified range type column (Table 1). The first 10 listings for this state in Appendix A do not specify the type of range (native or non-native) it is assigned to. The first specific type of range is given by Hobbs (1972), and this author considered the rusty crayfish to be a native species in Illinois. The first study to include Illinois in the non-native range of F. rusticus was the one by Page (1985). Taylor (2000) considered the rusty crayfish to be native to southwestern Illinois and non-native in the rest of the state. It could be argued that this crayfish may be native to a part or parts of Illinois and non-native in the rest of the state. However, the study by Taylor (2000) is the only one which places Illinois in both the native and the non-native range categories. All other studies listed in Appendix A which mention Illinois place this state either in the native range category or in the non-native range category.
Tennessee
This state has been included 17 times in the native range and 11 times in the non-native range of the rusty crayfish. There were also 15 listings in the unspecified range-type column, including the first 12 entries for this state in Appendix A. The first specific type of range is given by Hobbs (1974), who considered the rusty crayfish to be native to Tennessee. Crocker & Barr (1968) agreed with this assessment by Hobbs (1974), but Hamr (1998) and Lodge et al. (2000a) disagreed and included Tennessee in the non-native range of F. rusticus. David Lodge and researchers working in his lab were very influential in getting the rusty crayfish recognized as an invasive species and spreading this notion in the specialized literature, based mainly on studies conducted in some northern Wisconsin lakes (for example, Olsen et al., 1991; DiDonato & Lodge, 1993; Kershner & Lodge, 1995; Hill & Lodge, 1999).
The situation in Wisconsin seems somewhat more clear, since the vast majority of listings for this state (108) are in the non-native range column. The high number of studies in this category reflects, at least in part, the high level of research interest in the rusty crayfish as an invasive species in Wisconsin. However, there was also one listing for this state in the native range column, and 14 listings did not specify the type of range (Table 1). The first seven entries for this state in Appendix A did not specify the type of range (native or non-native). The first specific type of range was given by Capelli (1975), who included Wisconsin in the non-native range of the rusty crayfish. This was followed by Lorman (1975) who cited Capelli (1975), but listed Wisconsin as a part of both the non-native and the native range of F. rusticus.
The other five states (Iowa, Minnesota, Missouri, West Virginia, and Virginia) which appear in both the native and the non-native range columns have comparatively low numbers of listings overall. Iowa, Minnesota, and West Virginia are more often considered to be part of the non-native range of the rusty crayfish, whereas Missouri and Virginia appear slightly more often in the native range column than in the non-native range one (Table 1). To complicate matters further, Wetzel et al. (2004) conducted field work and examined relevant museum specimens and determined that at least some records from Iowa and Minnesota previously attributed to F. rusticus actually belong to the similar species Faxonius luteus (Creaser, 1933), which is regarded as a native species in these two states. Based on these observations, Wetzel et al. (2004) suggested that the ranges of both F. rusticus and F. luteus should be reassessed, not only in Iowa and Minnesota, but also perhaps in parts of Wisconsin and Illinois, where some F. luteus specimens may have been mistakenly identified as F. rusticus.
Twenty of the remaining 23 U.S. states listed in Table 1 appear either in the native range column or in the non-native range column, but never in both of these columns. Two of these states (Indiana and Kentucky) have plenty of listings in the native range column (55, for each of these two states), and some listings in the unspecified type of range column (19 in each case), and can therefore be reliably included in the native range of the rusty crayfish based on current knowledge. The same pattern applies to Alabama, although there are few listings for this state (2 in the native range column and 1 in the unspecified type of range column). There was only one listing for Mississippi, which has been included as part of the native range of the rusty crayfish by Crocker & Barr (1968). However, since this is the only record from Mississippi in our data set, and given the fact that Crocker & Barr (1968) focused on the crayfish of Ontario, and referred to several subspecies of F. rusticus in their analysis, caution should be exercised in interpreting this listing. It is quite possible that the lone record of this species for Mississippi may in fact be reassessed and attributed to another similar and closely related crayfish species.
Species identification can be challenging for crayfish species, and mistakes can often be made (Guiaşu, 2016). Taylor (2000) assigned F. rusticus to the Faxonius juvenilis (Hagen, 1870) species complex, which contains several closely related and very similar-looking species. The exact composition of this species complex was debated for more than 100 years, and, at various times, several species within this complex—such as, for example, F. juvenilis—were considered synonymous with F. rusticus. Since the differences between these species are very subtle, and all these species occupy similar habitats, it is often difficult to tell these species apart, and this creates additional problems for the assessment of their distributions.
Sixteen states (Pennsylvania, New Mexico, New York, Massachusetts, Maine, New Hampshire, Vermont, Connecticut, North Carolina, New Jersey, Oregon, Utah, Colorado, Nebraska, Washington, and Wyoming) never appear in the native range column for the rusty crayfish, and are listed only in the non-native range column or in both the non-native range and the unspecified type of range columns. As can be seen, these states are generally found in the northeastern and western areas of the U.S.A., often relatively far away from the presumed midwestern center of origin for this crayfish species. Therefore, at least some of these 16 U.S. states—for example those, such as New Mexico or Washington, not adjacent to regions where the rusty crayfish is considered to be native—can probably be safely assumed to be part of the non-native range of F. rusticus.
For the remaining 3 U.S. states (Texas, Arkansas, and Kansas) listed in Table 1, we have relatively few distribution records for the rusty crayfish and none of these records specify the type of range. These records were also published long ago, either in the late nineteenth century or early in the twentieth century, before a clear focus on native and non-native ranges became fashionable. For example, the 5 records for Texas were published in 1884, 1885, 1886, 1889, and 1912, respectively, the 3 records for Arkansas were published in 1885, 1898, and 1912, respectively, and the 3 records for Kansas were published in 1890, 1912, and 1931, respectively. Given that the presence of F. rusticus has never been reported in these states since then, it is possible that these early records may have been erroneous due to the misidentification of the crayfish species. It is also theoretically possible that the rusty crayfish may have been present in these 3 states many decades ago, but disappeared from these locations since then.
Ontario is the only Canadian Province included in both the native range and the non-native range of F. rusticus. This province is listed 10 times in the native range column, 66 times in the non-native range column, and 18 times in the unspecified range-type column (Table 1). In their detailed book on the crayfishes of Ontario, which remains the most influential work of its type in the field for this region, Crocker & Barr (1968) were the first to include this province into a specific (native or non-native) type of range. These authors regarded the rusty crayfish as non-native in Ontario. The book by Crocker & Barr (1968) was important in establishing the notion that the rusty crayfish was non-native in Ontario. However, Hobbs (1972) disagreed, and was the first to refer to F. rusticus as a native species in Ontario. Jezerinac et al. (1995) thought that the rusty crayfish was native to southern Ontario, but non-native in northwest Ontario. The other two Canadian Provinces where F. rusticus was found—Manitoba and Quebec—were only included in the non-native range for this species.
Overall, 26 U.S. states and 3 Canadian Provinces were included in the non-native range of the rusty crayfish, and 14 U.S. states and 1 Canadian province were included in the native range of this species. The reported non-native range for this species is considerably larger than its native range (Table 1).
Interestingly, the first 32 studies mentioning the rusty crayfish, which are listed in Appendix A and were published between 1852 and 1942 (during a period of 90 years), did not refer specifically to either native or non-native ranges. Thirty-one of these early studies offered information on the rusty crayfish range without specifying whether this range was native or non-native, and the remaining study did not discuss range at all. The first study listed in Appendix A which mentioned native and non-native ranges for F. rusticus was the one by Rhoades (1944a). Then, the next 20 studies in Appendix A did not discuss specifically either the native or the non-native range. The next work to refer to the native and non-native ranges of the rusty crayfish was the previously discussed book by Crocker & Barr (1968). Therefore, during the 116 years between 1852 and 1968, only two studies referred specifically to native and non-native ranges for this crayfish species. This suggests that concepts such as native and non-native range were not considered nearly as important during the second half of the nineteenth century and the first half of the twentieth century as they are today. With the rise of invasion biology, particularly in the last 30 years or so, reports about the distributions of species tended to focus more and more on whether these distributions were perceived as “native” or not. As a result, the percentage of studies which mentioned the rusty crayfish range without identifying this range as either native or non-native, out of the total number of studies listing the distribution of this species, declined from nearly 57% for the 1852–1989 period to less than 25% for the 1990–2018 period.
As shown in the previous section, the average number of studies per year which mentioned the rusty crayfish increased tenfold during the 1990–2018 period, in comparison to the 1852–1989 period. This dramatic increase may be attributed, at least in part, to the rise of invasion biology, which led to an enhanced focus on species perceived as invasive, such as F. rusticus. The data also showed that many more studies on the rusty crayfish mentioned the non-native range rather than the native range of this species, particularly since 1990, which suggests more of an emphasis on the non-native range, in keeping with an increased interest in the rusty crayfish because of its perceived invasive status in many areas.
In order for a species to be regarded as non-native, or even possibly invasive, the assumption is that the species expanded its range and reached new areas with human assistance. The fundamental problem with this assumption is that in many cases we simply do not know how various species, including F. rusticus, expanded their respective ranges. It is frequently assumed, for example, that the rusty crayfish was brought to various regions by fishermen who used this species as bait (Lodge, 1993a; Gunderson, 1995; Taylor, 2000; Marsden & Hauser, 2009). While this is certainly possible, no evidence is usually provided for such claims. In fact, Gunderson (1995) acknowledges that “there is no direct evidence” for the assertion that “anglers using crayfish as bait are thought to be the primary cause” for the spread of rusty crayfish in certain areas. Helgen (1990) stated that “There is no real information on human-caused dispersal of F. rusticus in Minnesota. Fishermen using crayfish as live bait have been implicated, but there is no data either on the extent of use of crayfish in northern and north central Minnesota (most fishermen use leeches and minnows), nor on the species of crayfish sold by live bait dealers in the state.”
Hamr (2010) mentions that F. rusticus “is thought to have been introduced into Ontario in the early 1960s, presumably by anglers from Ohio.” In other words, we do not really know how the rusty crayfish reached Ontario, but we assume, without evidence, that the Ohio fishermen must have brought these crayfish here about 60 years ago. This mere assumption validates potential and actual control programs against F. rusticus. In the same study, Hamr (2010) mentions various ways of managing this supposedly introduced crayfish species, including the eradication of new populations. Clearly, eradication would not be considered if the rusty crayfish would be regarded as a native species in Ontario.
F. rusticus is a resourceful crayfish species which can survive in a variety of freshwater aquatic habitats, including small ponds, large lakes, and various types of streams, and even, occasionally, in areas with mainly wet mud and little available surface water (Guiaşu & Guiaşu, 2003; Guiaşu, 2007, 2008, 2016, 2021). The rusty crayfish is also able to dig fairly deep and extensive burrows in the banks of streams with clay substrate (Hamr, 1998). This species can dig burrows both in the field and in the lab, and has been observed to burrow in a variety of locations, for example in Ohio and Kentucky (Berrill & Chenoweth, 1982).
This is a crayfish species that is perfectly capable of dispersing itself efficiently without human help (Guiaşu, 2007, 2008, 2016). Fitzpatrick (1987) stated that “man has helped O. (P.) rusticus in its invasions, but much of the range represents its own vigorous and successful expansion into areas breaking free of ice cover.” In other words, following the end of the last Ice Age, the expansion of the range of this species in North America was mostly achieved by natural means, and we should not punish F. rusticus for being good at dispersing itself while also occasionally taking advantage, as other species do as well, of certain human-made changes to the environment. There is no reason to believe that the post-glaciation northern expansion of the ranges of a variety of species, including crayfish, on the North American continent should not continue (Guiaşu et al., 1996; Guiaşu, 2016). Fitzpatrick (1987) also concluded that the exact ways in which F. rusticus may have expanded its range “will probably always remain unknown.”
The rusty crayfish is generally considered to be a non-native, and even invasive, species in the Great Lakes of North America region, and is usually discriminated against in this region as a result (Lodge et al., 2000; Olden et al., 2006; Olden et al., 2009; Johnson et al., 2009; Larson et al., 2017; Crossman et al., 2018; Cole et al., 2018, and many others). However, it is worth noting that this crayfish species is also regarded by many researchers as native to some states—such as Indiana, Ohio, Michigan, and Illinois—which border the Great Lakes (Table 1 and Appendix A). While referring to the rusty crayfish as “a nasty invader,” Gunderson (1995) acknowledges that this species “is native to parts of some Great Lakes states.” It is certainly possible to entertain the notion that F. rusticus may use its adaptability to various types of habitats and its superior dispersal abilities to expand its range gradually, and entirely on its own, through a network of interconnected rivers and tributaries all the way to the Great Lakes, and then within the vast Great Lakes freshwater system (Guiaşu, 2016). Peters et al. (2014) stated that the rusty crayfish was present in Lake Erie for more than a century, and may have been “originally native to southwestern Lake Erie.” If that is indeed the case, then there would be nothing to prevent F. rusticus from expanding its range into the other Great Lakes, and some of the streams and lakes adjacent to the Great Lakes (Guiaşu, 2016). Despite this, and the fact that the first record of the rusty crayfish from Lake Erie was from 1897, Peters et al. (2014) still label F. rusticus as a non-native species in all of the Great Lakes. This raises another question. For how long does a species have to be present at a certain location before we can finally accept it as a “natural” part of local ecosystems and leave it alone? Is a period of 123 years—the minimum length of time spent by the rusty crayfish in Lake Erie (as far as we know)—enough? These are the types of important questions invasion biologists generally do not seem particularly concerned about (Guiaşu, 2016). Jernelöv (2017) reviewed this topic in a recent book and found that “in most countries, there is no official policy with regard to the length of time a species has to be present before it more or less automatically receives citizenship or is considered native” and “where policies exist, they can differ significantly.” For example, according to Jernelöv (2017), federal law in Germany gives native status to any animal or plant species that can survive in the wild without human help for several generations, regardless of whether that species has been initially introduced by humans or not. However, in several other countries, species suspected of having arrived with human assistance are not officially accepted as native, or a natural part of local ecosystems, even after several decades or centuries. For instance, the common carp (Cyprinus carpio Linnaeus, 1758)—a well-known freshwater fish considered native to parts of Europe and Asia—is still regarded as an invasive species in Britain, even though it was introduced there before 1496 (Booy et al., 2015). This species is a valuable food fish, which was also successfully introduced into the U.S.A. in 1877 by the U.S. Fish Commission. Despite the fact that the common carp has been present in North America for 143 years, this fish is still not accepted by many as a truly local species on this continent (Guiaşu, 2016).
However, unlike the common carp, and other freshwater species which have been brought to North America from Europe and Asia by people, the rusty crayfish has originated and continued to evolve on the North American continent. Huston (1994) defined invaders as “species that have spread far beyond their original distribution, to distant continents or sometimes around the entire globe.” This definition does not seem to apply to the spread of the rusty crayfish in North America, at least in those regions adjacent to the presumed original, and poorly known, native range of this species. F. rusticus is accused of displacing the congeners Faxonius propinquus (Girard, 1852) and Faxonius virilis (Hagen, 1870) in some regions of North America—notably in Wisconsin and Ontario (Lodge, 1993a; Hamr, 1998). However, these three crayfish species are ecologically similar and have similar life cycles, diets, and habitat preferences (Lodge, 1993a). Furthermore, all three crayfish species are native to North America, where they remain quite common and very widely distributed, and they have broadly overlapping “natural” ranges. Thus, it would be hard to make the case that F. rusticus had a very different evolutionary history than these other closely related North American crayfish species. Lodge (1993a) reviewed the changing distributions and population dynamics of these three species in some northern Wisconsin lakes during the twentieth century, and noted that F. virilis was the most abundant crayfish in that region in 1930, but was then partially displaced by F. propinquus, which likely arrived in the 1950s, and then by F. rusticus, which reached the area probably in the 1960s and is capable of displacing the other two congeners in these lakes. Lodge (1993a) refers to the arrivals of both F. propinquus and F. rusticus in northern Wisconsin as “invasions from the southern Midwest” of the U.S.A., but these types of distribution and species composition changes can also be regarded as normal and should be expected over time. Newly arrived crayfish species may establish themselves successfully in certain regions mainly because they are better adapted to the changing local environmental conditions (Guiaşu, 2009). Interestingly, both F. virilis and F. propinquus are also perceived as “invasive” species when they expand their presumed native ranges further, to other regions of North America. For example, F. propinquus is considered native to southern and eastern Wisconsin but invasive in the northern part of that state, and the western range expansion of F. virilis in North America is also labeled as an “invasion” (Olden et al., 2006; Phillips et al., 2009).
The distributions of species are, of course, dynamic, and we cannot expect species to remain forever within the boundaries of the ranges we rather arbitrarily designated as “native” on their behalf (Guiaşu, 2016). There must have been many changes in the distributions of crayfish species in eastern and central North America since the end of the last Ice Age, approximately 10,000 years ago, and we can expect more of these changes in the future as well, of course. Trying to stop or minimize potential range expansions by some of these crayfish species on their native continent, especially in areas adjacent to their presumed native ranges, and in the absence of clear information about means of dispersal, seems to be a rather dubious and largely futile exercise. Such attempts to control crayfish species perceived as “invasive” may also have unintended consequences and negative effects on other species (Guiaşu, 2016). For example, chemicals which kill the rusty crayfish will also inevitably kill other crayfish species as well, including presumably native species (Gunderson, 1995; Olden et al., 2006). Also, given the previously discussed similarities among closely related crayfish species in North America, and the fact that even experts often have a tough time telling such species apart, enlisting the help of the public to remove rusty crayfish from various surface waters would probably lead to the destruction of several other crayfish species as well in the targeted areas. There are probably better ways to spend the limited conservation-related resources available (Guiaşu, 2016; Guiaşu & Tindale, 2018).
It should also be mentioned that sometimes changes in environmental conditions can lead to major declines in populations of both native and non-native crayfish species, including F. rusticus. For example, Edwards et al. (2009) found significant and geographically widespread population declines for all the seven crayfish species (native and non-native) sampled during a major survey of 100 lakes from a large region of south-central Ontario. This major decline occurred since the early 1990s and led to less diverse crayfish species assemblages in these lakes and much reduced or lost populations of all crayfish species in this region, including F. propinquus and F. virilis, which are considered native in Ontario, and the supposedly invasive F. rusticus. The rusty crayfish was rare in this region, and local populations of this species declined by 91% during this period of time. Overall, Edwards et al. (2009) stated that they “found no evidence to indicate that the presence of non-native crayfish was related to the decline of native species” and concluded that F. rusticus “was not a factor in the declines” of all these crayfish species in Ontario.
Our knowledge of historical North American crayfish distributions is often based on relatively few museum specimens, the oldest of which were usually collected in the late nineteenth century or the early twentieth century. For example, the oldest crayfish museum records in Ontario are from the early 1900s and are very incomplete. As a result, almost nothing is known about the distributions of crayfish species in this Canadian Province before the early twentieth century, and the relatively few records collected since then are not sufficient to give us a comprehensive picture of how or why these distributions may have changed over time (Guiaşu, 2016, 2021; Pereyra & Guiaşu, 2020).
Even assuming that F. rusticus may have made it to the Great Lakes with the aid of people back in the nineteenth century, or even earlier, it certainly seems like a long time has gone by since this crayfish species has lived in and around at least some of the Great Lakes, and there have been many irreversible changes to the habitats and species compositions of this vast region during all this time.
It is also entirely possible—indeed, likely—that the rusty crayfish may disperse itself both with and without human assistance, and, in any event, it is very difficult to differentiate naturally dispersing populations of such species from conspecific populations which benefit from direct or indirect human activities (Guiaşu, 2016; Pereyra & Guiaşu, 2020). Even in an article focused mainly on the invasion history of F. rusticus in Wisconsin, Olden et al. (2006) had to admit that “natural inter-lake dispersal of rusty crayfish is also possible and may represent an important vector of introduction” in that state. The problem is that, once a species such as the rusty crayfish is declared to be non-native, and especially invasive, in particular regions, this species is always likely to be viewed negatively by local conservation biologists and managers even in the absence of conclusive evidence for its non-native status, and even if the species may well be able to reach these regions by a variety of means, including “natural” (or nothuman assisted) ones. So, for example, although the rusty crayfish can probably reach Ontario on its own from nearby regions—such as Michigan and Ohio—where it is considered by many to be native, and may have already done this several times since the last Ice Age, this species is likely to be always considered invasive in this Canadian Province, simply because of the assumption that it may have been once brought here by people (Guiaşu, 2016).
The debates about the exact extent of the native range of F. rusticus continue. For example, in a fairly recent study, Dresser & Swanson (2013) presented two alternative possible native ranges for this crayfish species. According to the first version, attributed to the researchers of The United States Geological Survey, the rusty crayfish is native to Indiana, Illinois, Kentucky, Ohio, and Tennessee. However, the other native range given in this same study for F. rusticus encompasses Ohio, Kentucky, Indiana, and Michigan. According to Dresser & Swanson (2013), this second native range is “accepted by researchers of invasive crayfish.” Based on what we know from the investigation of the relevant literature, this statement seems overly general, vague, and quite optimistic. Perhaps some researchers in this field may agree on this rather limited version of the native range of the rusty crayfish, but certainly not all. In support of the statement that researchers of invasive crayfish accept this particular version of the native range, Dresser & Swanson (2013) cite three studies: Taylor, 2000; DiStefano et al., 2009; and Peters & Lodge, 2009. As it turns out, however, none of these studies deal primarily with this revised version of the rusty crayfish’s range. Furthermore, in one of these studies, Taylor (2000) mentions “a less than clear understanding” of the ranges of several crayfish species, including F. rusticus, and admits that few specimens of this species from outside Kentucky were looked at, and the range of the rusty crayfish in states adjacent to Kentucky “is far from being completely known.” While, as previously discussed, it seems fairly safe to include Indiana and Kentucky within the native range of F. rusticus, and the Dresser & Swanson (2013) article appears to confirm that, many controversies about the exact status of this species in several other U.S. states, or regions of states, remain.
It is important to recognize and analyze the uncertainties about the history, extent, and limits of the native ranges of species such as the rusty crayfish, because determining native or non-native status for these species may have important implications for the ways in which the species are perceived and treated. Native species are often protected and viewed positively while non-native ones may become the subject of control and even attempted eradication programs (Guiaşu, 2016; Pereyra & Guiaşu, 2020). This may lead to the development of bias in ecological and conservation studies focused on non-native species, and an emphasis on the perceived negative impacts of these species, while ignoring or minimizing their potential positive contributions to ecosystems (Schlaepfer et al., 2011; Guiaşu, 2016; Guiaşu & Tindale, 2018). Thus, estimating the native or non-native status of species can be a highly subjective exercise, often based on incomplete and/or contradictory information. At the same time, assessments of the ecological impacts of species can also be quite subjective, and may be influenced by the perception of the native or non-native status of these species (Guiaşu, 2016).
The determination of the native or non-native status of the rusty crayfish may have an important role for the interpretation of the data in other types of studies—for example, behavioral and physiological studies as well. For instance, Hazlett et al. (2002) compared the memory capabilities of invasive and native crayfish species. Two of the paired species chosen for the study were Faxonius rusticus, as the invasive species in Michigan, and Faxonius virilis, as the native species in that same state. The study concluded that the supposedly invasive F. rusticus retained information longer than the native F. virilis, and on the basis of this conclusion, the authors offered the generalization that invasive species show greater behavioral plasticity than native species, which presumably gives the invasive species an advantage over the native ones. However, if F. rusticus turns out to be a native species in Michigan after all, as quite a few studies have stated (Table 1), then the general conclusion of the Hazlett et al. (2002) study would have to be reassessed.
Conclusion
F. rusticus has been intensely studied, particularly in the last few decades, undoubtedly at least in part because of its perceived invasive status. Given the on-going debates and continuing controversies regarding the extent and history of the native range of the rusty crayfish, caution should be exercised and more conclusive evidence should be gathered, before making definitive pronouncements about the native or non-native status of this species at many locations. It is important to acknowledge that often we simply do not have enough information about the distributions of species, and, in many cases, the crucial relevant facts which would allow us to confidently decide whether a species is native or not (assuming we think such a distinction matters) may never become available.
References
Acquistapace, P., B. A. Hazlett & F. Gherardi, 2003. Unsuccessful predation and learning of predator cues by crayfish. Journal of Crustacean Biology 23: 364–370.
Adams, J. A. & P. A. Moore, 2003. Discrimination of conspecific male molt odor signals by male crayfish, Orconectes rusticus. Journal of Crustacean Biology 23: 7–14.
Alberstadt, P. J., C. W. Steele & C. Skinner, 1995. Cover-seeking behavior in juvenile and adult crayfish, Orconectes rusticus: effects of darkness and thigmotactic cues. Journal of Crustacean Biology 15: 537–541.
Alberstadt, P., C. Steele, K. Misra, C. Skinner, B. Wilson & S. Robaskiewicz, 1999. Sublethal exposure to cadmium on shelter-seeking behavior of juvenile Orconectes rusticus (Girard) crayfish. Toxicology and Risk Assessment 8: 362–369.
Albertson, L. K. & M. D. Daniels, 2016. Effects of invasive crayfish on fine sediment accumulation, gravel movement, and macroinvertebrate communities. Freshwater Science 35: 644–653.
Alexander, M. L., M. P. Woodford & S. S. Hotchkiss, 2008. Freshwater macrophyte communities in lakes of variable landscape position and development in northern Wisconsin, U.S.A. Aquatic Botany 88: 77–86.
Anderson, W. E. & T. P. Simon, 2015. Length-weight relationship, body morphometrics, and condition based on sexual stage in the rusty crayfish, Orconectes rusticus Girard, 1852 (Decapoda, Cambaridae) with emphasis on management implications. Fisheries and Aquaculture Journal 6: 129–135.
Arcella, T. E., W. L. Perry, D. M. Lodge & J. L. Feder, 2014. The role of hybridization in a species invasion and extirpation of resident fauna: hybrid vigor and breakdown in the rusty crayfish, Orconectes rusticus. Journal of Crustacean Biology 34: 157–164.
Baldridge, A. K. & D. M. Lodge, 2013. Intraguild predation between spawning smallmouth bass (Micropterus dolomieu) and nest-raiding crayfish (Orconectes rusticus): implications for bass nesting success. Freshwater Biology 58: 2355–2365.
Baldridge, A. K. & D. M. Lodge, 2014. Long-term studies of crayfish-invaded lakes reveal limited potential for macrophyte recovery from the seed bank. Freshwater Science 33: 788–797.
Barkman, R. C., 1970. Response of the tissue of Orconectes rusticus to salinity stress. Comparative Biochemistry and Physiology 36: 285–290.
Barr, D., 1969. The crayfish: fresh-water giant. Canadian Audubon 31: 92–94.
Bauke, Z, 2018. Effects of Faxonius rusticus on erosion in a lotic ecosystem. Undergraduate dissertation. Carthage College, Kenosha, WI.
Bazer, C. E., R. L. Preston & W. L. Perry, 2016. Increased salinity affects survival and osmotic response of rusty crayfish Orconectes rusticus and northern clearwater crayfish O. propinquus (Decapoda: Astacoidea: Cambaridae) as salinity increases: the potential for estuarine invasions. Journal of Crustacean Biology 36: 607–614.
Beattie, M. C. & P. A. Moore, 2018. Predator recognition of chemical cues in crayfish: diet and experience influence the ability to detect predation threats. Behaviour 155: 505–530.
Belanger, J. H., 1988. Temperature acclimation of the caudal photoreceptor response in the crayfish Orconectes rusticus (Girard). Canadian Journal of Zoology 66: 1168–1171.
Belanger, R. M. & P. A. Moore, 2006. The use of the major chelae by reproductive male crayfish (Orconectes rusticus) for discrimination of female odours. Behaviour 143: 713–732.
Belanger, R. M. & P. A. Moore, 2009. The role of the major chelae in the localization and sampling of female odours by male crayfish, Orconectes rusticus (Girard, 1852). Crustaceana 82: 653–668.
Belanger, R. M. & P. A. Moore, 2013. A comparative analysis of setae on the pereiopods of reproductive male and female Orconectes rusticus (Decapoda: Astacidae). Journal of Crustacean Biology 33: 309–316.
Belanger, R. M., X. Ren, K. McDowell, S. Chang, P. A. Moore & B. Zielinski, 2008. Sensory setae on the major chelae of male crayfish, Orconectes rusticus (Decapoda: Astacidae)—impact of reproductive state on function and distribution. Journal of Crustacean Biology 28: 27–36.
Belanger, R. M., T. J. Peters, G. S. Sabhapathy, S. Khan, K. Katta & N. K. Abraham, 2015. Atrazine exposure affects the ability of crayfish (Orconectes rusticus) to localize a food odor source. Archives of Environmental Contamination and Toxicology 68: 636–645.
Belanger, R. M., K. R. Evans, N. K. Abraham & K. M. Barawi, 2017. Diminished conspecific odor recognition in the rusty crayfish (Orconectes rusticus) following a 96-h exposure to atrazine. Bulletin of Environmental Contamination and Toxicology 99: 555–560.
Bergman, D. A. & P. A. Moore, 2003. Field observations of intraspecific agonistic behavior of two crayfish species, Orconectes rusticus and Orconectes virilis, in different habitats. The Biological Bulletin 205: 26–35.
Bergman, D. A. & P. A. Moore, 2005. Prolonged exposure to social odours alters subsequent social interactions in crayfish (Orconectes rusticus). Animal Behaviour 70: 311–318.
Bergman, D. A., A. L. Martin & P. A. Moore, 2005. Control of information flow through the influence of mechanical and chemical signals during agonistic encounters by the crayfish, Orconectes rusticus. Animal Behaviour 70: 485–496.
Bergman, D. A., C. N. Redman, K. C. Fero, J. L. Simon & P. A. Moore, 2006. The impacts of flow on chemical communication strategies and fight dynamics of crayfish. Marine and Freshwater Behaviour and Physiology 39: 245–258.
Bernot, R. J. & A. M. Turner, 2001. Predator identity and trait-mediated indirect effects in a littoral food web. Oecologia 129: 139–146.
Berrill, M., 1978. Distribution and ecology of crayfish in the Kawartha Lakes region of southern Ontario. Canadian Journal of Zoology 56: 166–177.
Berrill, M., 1985. Laboratory induced hybridization of two crayfish species, Orconectes rusticus and O. propinquus. Journal of Crustacean Biology 5: 347–349.
Berrill, M. & M. Arsenault, 1982. Spring breeding of a northern temperate crayfish, Orconectes rusticus. Canadian Journal of Zoology 60: 2641–2645.
Berrill, M. & M. Arsenault, 1984. The breeding behaviour of a northern temperate orconectid crayfish, Orconectes rusticus. Animal Behaviour 32: 333–339.
Berrill, M. & D. Chenoweth, 1982. The burrowing ability of nonburrowing crayfish. The American Midland Naturalist 108: 199–201.
Berrill, M., L. Hollett, A. Margosian & J. Hudson, 1985. Variation in tolerance to low environmental pH by the crayfish Orconectes rusticus, O. propinquus, and Cambarus robustus. Canadian Journal of Zoology 63: 2586–2589.
Berry, B. O., 1980. A comparative study of the uptake of toluene by bluegill sunfish Lepomis macrochirus and crayfish Orconectes rusticus. Environmental Pollution Series A, Ecological and Biological 21: 109–119.
Bhimany, R. & R. Huber, 2016. Operant avoidance learning in crayfish, Orconectes rusticus: computational ethology and the development of an automated learning paradigm. Learning & Behavior 44: 239–249.
Bills, T. D. & L. L. Marking, 1988. Control of nuisance populations of crayfish with traps and toxicants. The Progressive Fish-Culturalist 50: 103–106.
Bobeldyk, A. M. & G. Lamberti, 2008. A decade after invasion: evaluating the continuing effects of rusty crayfish on a Michigan river. Journal of Great Lakes Research 34: 265–275.
Bobeldyk, A. M. & G. A. Lamberti, 2010. Stream food web responses to a large omnivorous invader, Orconectes rusticus (Decapoda, Cambaridae). Crustaceana 83: 641–657.
Booy, O., M. Wade & H. Roy, 2015. Field Guide to Invasive Plants and Animals in Britain. Bloomsbury, London.
Bouchard, R. W., 1977. Distribution, ecology, and systematic status of five poorly known western North American crayfishes (Decapoda: Astacidae and Cambaridae). Freshwater Crayfish 3: 409–423.
Bouchard, R. W., 1978. Taxonomy, distribution, and general ecology of the genera of North American crayfishes. Fisheries 3: 11.
Bouchard, R. W. & J. W. Bouchard, 1995. Two new species and subgenera (Cambarus and Orconectes) of crayfishes (Decapoda: Cambaridae) from the eastern United States. The Academy of Natural Sciences of Philadelphia.
Bouchard, R. W., D. A. Lieb, R. F. Carline, T. R. Nuttall, C. B. Wengert & J. R. Wallace, 2007. 101 years of change (1906 to 2007). The distribution of the crayfishes of Pennsylvania. Part I. Eastern Pennsylvania. Academy of Natural Sciences of Philadelphia 7: 39.
Brazner, J. C. & D. A. Jensen, 2000. Zebra mussel [Dreissena polymorpha (Pallas)] colonization of rusty crayfish [Orconectes rusticus (Girard)] in Green Bay, Lake Michigan. The American Midland Naturalist 143: 250–257.
Brown, P. L., 1955. The biology of the crayfishes of central and southeastern Illinois. Doctoral dissertation. University of Illinois at Urbana-Champaign, Champaign County, IL.
Brown, K. M., 1998. The role of shell strength in selective foraging by crayfish for gastropod prey. Freshwater Biology 40: 255–260.
Browne, A. M. & P. A. Moore, 2014. The effects of sublethal levels of 2,4-dichlorophenoxyacetic acid herbicide (2,4-d) on feeding behaviors of the crayfish O. rusticus. Archives of Environmental Contamination and Toxicology 67: 234–244.
Brown, P. R., M. R. White, D. L. Swann & M. S. Fuller, 1993. A severe outbreak of ectoparasitism due to Epistylis sp. in pond-reared orconectid crayfish. Journal of the World Aquaculture Society 24: 116–120.
Bruski, C. A. & D. W. Dunham, 1987. The importance of vision in agonistic communication of the crayfish Orconectes rusticus. I: an analysis of bout dynamics. Behaviour 103: 83–107.
Bruski, C. A. & D. W. Dunham, 1990. Antennal waving in the crayfish Orconectes rusticus (Girard, 1852) (Decapoda, Astacidea). Crustaceana 58: 83–87.
Bundy, W. F., 1882. A list of the Crustacea of Wisconsin, with notes on some new or little known species. Transactions on the Wisconsin Academy of Sciences 5: 177–184.
Butler, M. J., 1982. Interspecific displacement of crayfish: analysis of the competitive mechanism. The Bulletin of the Ecological Society of America 68: 113.
Butler, M. J., 1988. Evaluation of possible reproductively mediated character displacement in the crayfishes, Orconectes rusticus and O. sanbornii. The Ohio Journal of Science 88: 87–91.
Butler, M. J. & R. A. Stein, 1985. An analysis of the mechanisms governing species replacements in crayfish. Oecologia 66: 168–177.
Byron, C. J. & K. A. Wilson, 2001. Rusty crayfish (Orconectes rusticus) movement within and between habitats in Trout Lake, Vilas County, Wisconsin. Journal of the North American Benthological Society 20: 606–614.
Callaghan, D. T., W. A. Dew, C. D. Weisbord & G. G. Pyle, 2012. The role of various sensory inputs in establishing social hierarchies in crayfish. Behaviour 149: 1443–1458.
Capelli, G. M., 1975. Distribution, life history, and ecology of crayfish in northern Wisconsin, with emphasis on Orconectes propinquus. Doctoral dissertation. University of Wisconsin-Madison, Madison, WI.
Capelli, G. M., 1982. Displacement of northern Wisconsin crayfish by Orconectes rusticus (Girard). Limnology and Oceanography 27: 741–745.
Capelli, G. M. & J. F. Capelli, 1980. Hybridization between crayfish of the genus Orconectes: morphological evidence (Decapoda, Cambaridae). Crustaceana 39: 121–132.
Capelli, G. M. & P. A. Hamilton, 1984. Effects of food and shelter on aggressive activity in the crayfish Orconectes rusticus (Girard). Journal of Crustacean Biology 4: 252–260.
Capelli, G. M. & J. J. Magnuson, 1983. Morphoedaphic and biogeographic analysis of crayfish distribution in Northern Wisconsin. Journal of Crustacean Biology 3: 548–564.
Capelli, G. M. & P. E. McIntire, 1980. Mating duration, sperm plug formation, and potential reproductive interference among three crayfish species of the genus Orconectes. Limnology & Oceanography.
Capelli, G. M. & B. L. Munjal, 1982. Aggressive interactions and resource competition in relation to species displacement among crayfish of the genus Orconectes. Journal of Crustacean Biology 2: 486–492.
Carpenter, S. R., B. J. Benson, R. Biggs, J. W. Chipman, J. A. Foley, S. A. Golding, R. B. Hammer, P. C. Hanson, P. T. J. Johnson, A. M. Kamarainen, T. K. Kratz, R. C. Lathrop, J. D. McMahon, B. Provencher, J. A. Rusak, C. T. Solomon, E. H. Stanley, M. G. Turner, M. J. Vander Zanden, C. Wu & H. Yuan, 2007. Understanding regional change: a comparison of two lake districts. Bioscience 57: 323–335.
Cava, Z. A., A. M. McMillan, C. M. Pennuto & R. J. Warren, 2018. Hellbender prey preference is superseded by native and nonnative prey behavior. Journal of Herpetology 52: 162–170.
Charlebois, P. M., 1994. The effects of crayfish (Orconectes rusticus) on the macroinvertebrate and algal assemblages in a northern Michigan stream. Master’s dissertation. University of Notre Dame, Notre Dame, IN.
Charlebois, P. M. & G. A. Lamberti, 1996. Invading crayfish in a Michigan stream: direct and indirect effects on periphyton and macroinvertebrates. Journal of the North American Benthological Society 15: 551–563.
Chibucos, K., S. J. Wofford & P. A. Moore, 2015. Hierarchical decision making: resource distribution exhibits stronger effect on crayfish dominance relationships and shelter occupation than prior social experience and resource ownership. Behaviour 152: 1063–1082.
Claramunt, R. M., J. L. Jonas, J. D. Fitzsimons & J. E. Marsden, 2005. Influences of spawning habitat characteristics and interstitial predators on lake trout egg deposition and mortality. Transactions of the American Fisheries Society 134: 1048–1057.
Clark, J. L. & P. A. Moore, 2018. The role of sensory modalities in producing non-consumptive effects for a crayfish-bass predator prey system. Canadian Journal of Zoology 96: 680–691.
Claussen, D. L., 1980. Thermal acclimation in the crayfish, Orconectes rusticus and O. virilis. Comparative Biochemistry and Physiology Part A: Physiology 66: 377–384.
Claussen, D. L., R. A. Hopper & A. M. Sanker, 2000. The effects of temperature, body size, and hydration state on the terrestrial locomotion of the crayfish Orconectes rusticus. Journal of Crustacean Biology 20: 218–223.
Cole, E., R. P. Keller & K. Garbach, 2018. Risk of invasive species spread by recreational boaters remains high despite widespread adoption of conservation behaviors. Journal of Environmental Management 229: 112–119.
Cook, M. E. & P. A. Moore, 2008. The effects of the herbicide metolachlor on agonistic behavior in the crayfish, Orconectes rusticus. Archives of Environmental Contamination and Toxicology 55: 94–102.
Cook, M. E. & P. A. Moore, 2009. Communication networks and loser effects interact to influence the outcome of aggressive interactions in the crayfish Orconectes rusticus. Behaviour 146: 263–281.
Cooper, J. E. & S. A. Armstrong, 2007. Locality records and other data for invasive crayfishes (Decapoda: Cambaridae) in North Carolina. Journal of the North Carolina Academy of Science 123: 1–13.
Corey, S., 1987. Comparative fecundity of four species of crayfish in Southwestern Ontario, Canada (Decapoda, Astacidea). Crustaceana 52: 276–286.
Corey, S., 1988. Comparative life histories of two populations of the introduced crayfish Orconectes rusticus (Girard, 1852) in Ontario. Crustaceana 55: 29–38.
Corey, S., 1991. Comparative potential reproduction and actual production in several species of North American crayfish. Crustacean Egg Production 7: 69–76.
Coulter, D. P., M. S. Sepúvelda, C. D. Troy & T. O. Höök, 2014. Thermal habitat quality of aquatic organisms near power plant discharges: potential exacerbating effects of climate warming. Fisheries Management and Ecology 21: 196–210.
Courchamp, F., P. E. Hulme & P. Pyšek, 2020. Invasion biology and uncertainty in native range definitions: response to Pereyra 2019. Conservation Biology 34: 1041–1043.
Crabtree, R. L. & R. G. Sherman, 1980. Innervation of the crayfish thoracic deep flexor musculature. Journal of Experimental Zoology 213: 15–24.
Crandall, K. A. & S. De Grave, 2017. An updated classification of the freshwater crayfishes (Decapoda: Astacidea) of the world, with a complete species list. Journal of Crustacean Biology 37: 615–653.
Creaser, E. P., 1931. The Michigan decapod crustaceans. Michigan Academy of Science, Arts and Letters 13: 257–276.
Creaser, E. P., 1932. The decapod crustaceans of Wisconsin. Transactions of the Wisconsin Academy of Sciences 27: 321–338.
Creed, R. P. & J. M. Reed, 2004. Ecosystem engineering by crayfish in a headwater stream community. Journal of the North American Benthological Society 23: 224–236.
Crocker, D. W., 1957. The crayfishes of New York State (Decapoda, Astacidae) (No. 355). University of the State of New York.
Crocker, D. W., 1979. The crayfishes of New England. Proceedings from the Biological Society of Washington 92: 225–252.
Crocker, D. W. & D. W. Barr, 1968. Handbook of the Crayfishes of Ontario. University of Toronto Press, Royal Ontario Museum.
Crossman, J. A., K. T. Scribner, P. S. Forsythe & E. A. Baker, 2018. Lethal and non-lethal effects of predation by native fish and an invasive crayfish on hatchery-reared age-0 lake sturgeon (Acipenser fulvescens Rafinesque, 1817). Journal of Applied Ichthyology 34: 322–330.
Daniels, R. A., 1998. Changes in the distribution of stream-dwelling crayfishes in the Schoharie Creek System, Eastern New York State. Northeastern Naturalist 5: 231–248.
Daniels, R. A., D. C. Murphy & M. W. Klemens, 2001. Orconectes neglectus is established in the Northeast. Northeastern Naturalist 8: 93–100.
Darwin, C., 1859. On the Origin of Species by Means of Natural Selection Or the Preservation of Favoured Races in the Struggle for Life. John Murray, London.
David, S. M., K. M. Somers, R. A. Reid & R. I. Ingram, 1997. Crayfish Species Assemblages in Softwater Lakes in Seven Tertiary Watersheds in South-Central Ontario. Ministry of Environment & Energy, Dorset, Ontario.
Davis, K. & R. Huber, 2007. Activity patterns, behavioural repertoires, and agonistic interactions of crayfish: a non-manipulative field study. Behaviour 144: 229–247.
Davis, M. A., K. Thompson & J. P. Grime, 2001. Charles Elton and the dissociation of invasion ecology from the rest of ecology. Diversity and Distributions 7: 97–102.
Davis, M. J., J. L. Purrenhage & M. D. Boone, 2012. Elucidating predator–prey interactions using aquatic microcosms: complex effects of a crayfish predator, vegetation, and atrazine on tadpole survival and behavior. Journal of Herpetology 46: 527–535.
Daws, A., R. Huber, D. Bergman, J. McIntyre, P. A. Moore & C. Kozlowski, 2003. Temporal dynamics and communication of winner-effects in the crayfish, Orconectes rusticus. Behaviour 140: 805–825.
Daws, A. G., K. Hock & R. Huber, 2011. Spatial structure of hierarchical groups: testing for processes of aggregation, clustering, and spatial centrality in crayfish (Orconectes rusticus). Marine and Freshwater Behaviour and Physiology 44: 209–222.
Dean, J. L., 1969. Biology of the Crayfish Orconectes causeyi and Its Use for Control of Aquatic Weeds in Trout Lakes, 24. U.S. Fish and Wildlife Service.
Desroches, J. F., L. P. Gagnon & I. Picard, 2014. L’invasion de l’écrevisse à taches rouges au lac Brome, en Montérégie. Le Naturaliste Canadien 138: 46–49.
DeVillez, E. J. & D. J. Fyler, 1986. Isolation of hepatopancreatic cell types and enzymatic activities in B cells of the crayfish Orconectes rusticus. Canadian Journal of Zoology 64: 81–83.
DiDonato, G. T. & D. M. Lodge, 1993. Species replacements among Orconectes crayfishes in Wisconsin Lakes: the role of predation by fish. Canadian Journal of Fisheries and Aquatic Sciences 50: 1484–1488.
DiStefano, R. J., M. E. Litvan & P. T. Horner, 2009. The bait industry as a potential vector for alien crayfish introductions: problem recognition by fisheries agencies and a Missouri evaluation. Fisheries 34: 586–597.
Dodds, W. K., C. T. Robinson, E. E. Gaiser, G. J. A. Hansen, H. Powell, J. M. Smith, N. B. Morse, S. L. Johnson, S. V. Gregory, T. Bell, T. K. Kratz & W. H. McDowell, 2012. Surprises and Insights from Long-Term Aquatic Data Sets and Experiments. Bioscience 62: 709–721.
Dorn, N. J. & J. M. Wojdak, 2004. The role of omnivorous crayfish in littoral communities. Oecologia 140: 150–159.
Dougherty, M. M., E. R. Larson, M. A. Renshaw, C. A. Gantz, S. P. Egan, D. M. Erickson & D. M. Lodge, 2016. Environmental DNA (eDNA) detects the invasive rusty crayfish Orconectes rusticus at low abundances. Journal of Applied Ecology 53: 722–732.
Dresser, C. & B. Swanson, 2013. Preemptive legislation inhibits the anthropogenic spread of an aquatic invasive species, the rusty crayfish (Orconectes rusticus). Biological Invasions 15: 1049–1056.
Dresser, C. L., M. L. Kuhlmann & B. J. Swanson, 2016. Variation in native crayfish agonistic response to the invasion of the rusty crayfish Orconectes rusticus. Journal of Crustacean Biology 36: 129–137.
Dubé, J. & J. F. Desroches, 2007. Les écrevisses du Québec. Ministère des ressources naturelles et de la faune, Direction de l’aménagement de la faune de l’Estrie, de Montréal et de la Montérégie, Longueuil.
Dubé, J., R. Pariseau & D. St-Hilaire, 2002. Première mention de l’écrevisse Orconectes rusticus (Girard) au Québec. Naturaliste Canadien 126: 45–47.
Eberly, W. R., 1954. Summary of the distribution of Indiana crayfishes, including new state and county records. Proceedings of the Indiana Academy of Science 64: 281–283.
Edwards, B. A., D. A. Jackson & K. M. Somers, 2009. Multispecies crayfish declines in lakes: implications for species distributions and richness. Journal of the North American Benthological Society 28: 719–732.
Edwards, B. A., V. R. E. Lewis, F. H. Rodd & D. A. Jackson, 2013. Interactive effects of calcium decline and predation risk on the potential for a continuing northward range expansion of the rusty crayfish (Orconectes rusticus). Canadian Journal of Zoology 91: 328–337.
Edwards, B. A., D. A. Jackson & K. M. Somers, 2015. Evaluating the effect of lake calcium concentration on the acquisition of carapace calcium by freshwater crayfish. Hydrobiologia 744: 91–100.
Edwards, D. D., K. L. Klotz & P. A. Moore, 2018. Exposure to sublethal ammonia concentrations alters the duration and intensity of agonistic interactions in the crayfish, Orconectes rusticus. Bulletin of Environmental Contamination and Toxicology 100: 189–194.
Eggleston, P. M., 1975. The energy requirements of Orconectes rusticus, and its ability to utilize various species of algae as food. Doctoral dissertation. Ohio State University, Columbus, OH.
Eggleston, P. M. & S. L. Lustick, 1981. The oxygen requirements of the crayfish, Orconectes rusticus. The Ohio Journal of Science 81: 92–94.
Ellis, M. M., 1919. The branchiobdellid worms in the collection of the United States National Museum, with descriptions of new genera and new species. Proceedings of the United States National Museum 55: 241–265.
Ellrott, B. J., J. E. Marsden, J. D. Fitzsimons, J. L. Jonas & R. M. Claramunt, 2007. Effects of temperature and density on consumption of trout eggs by Orconectes propinquus & O. rusticus. Journal of Great Lakes Research 33: 7–14.
Elton, C. S., 1958. The Ecology of Invasions by Animals and Plants. Methuen, London.
Engel, R. A., 1966. Accumulation and metabolism of DDT in the crayfish Orconectes rusticus (Girard). Doctoral dissertation. Ohio State University, Columbus, OH.
Essl, F., S. Bacher, P. Genovesi, P. E. Hulme, J. M. Jeschke, S. Katsanevakis, I. Kowarik, I. Kühn, P. Pyšek, W. Rabitsch & S. Schindler, 2018. Which taxa are alien? Criteria, applications, and uncertainties. BioScience 68: 496–509.
Etchison, L., S. J. Jacquelin, M. Allen & M. Pyron, 2012. Morphological variation of rusty crayfish Orconectes rusticus (Cambaridae) with gender and local scale spatial gradients. International Journal of Biology 4: 263–271.
Evans, M. L., 1980. Copper accumulation in the crayfish (Orconectes rusticus). Bulletin of Environmental Contamination and Toxicology 24: 916–920.
Faxon, W., 1884a. Descriptions of new species of Cambarus; to which is added a synonymical list of the known species of Cambarus and Astacus. Metcalf and Company.
Faxon, W., 1884b. On the so-called dimorphism in the genus Cambarus. American Journal of Science 27: 42.
Faxon, W., 1885. A revision of the Astacidae. Memoirs of the Museum of Comparative Zoology 10: 1–186.
Faxon, W., 1890. Notes on North American crayfishes, family Astacidae. Proceedings of the United States National Museum 12: 619–634.
Faxon, W., 1898. Observations on the Astacidae in the United States National Museum and in the Museum of Comparative Zoology: with descriptions of new species. Proceedings of the United States National Museum 20: 643–712.
Faxon, W., 1914. Notes on the crayfishes in the United States National Museum and the Museum of Comparative Zoölogy with descriptions of new species and subspecies to which is appended a catalogue of the known species and subspecies. Memoirs of the Museum of Comparative Zoology at Harvard College 40: 384–385.
Fero, K., J. L. Simon, V. Jourdie & P. A. Moore, 2007. Consequences of social dominance on crayfish resource use. Behaviour 144: 61–82.
Fitzpatrick Jr., J. F., 1987. The subgenera of the crawfish genus Orconectes (Decapoda: Cambaridae). Proceedings of the Biological Society of Washington 100: 44–74.
Flemming, R. H., 1939. The larger Crustacea of the Nashville region. Journal of the Tennessee Academy of Science 13: 296–314.
Flynn, M. F. & H. H. Hobbs, 1984. Parapatric crayfishes in southern Ohio: evidence of competitive exclusion? Journal of Crustacean Biology 4: 382–389.
Forbes, S. A., 1884. List of Illinois Crustacea: with descriptions of new species. Bulletin of the Illinois Museum of Natural History 1: 3–16.
Forsythe, P. S., J. A. Crossman, C. P. Firkus, K. T. Scribner & E. A. Baker, 2018. Effects of crayfish density, body size and substrate on consumption of lake sturgeon (Acipenser fulvescens Rafinesque, 1817) eggs by invasive rusty crayfish [(Orconectes rusticus (Girard, 1852)]. Journal of Applied Ichthyology 34: 314–321.
Foster, H. R. & T. A. Keller, 2011. Flow in culverts as a potential mechanism of stream fragmentation for native and nonindigenous crayfish species. Journal of the North American Benthological Society 30: 1129–1137.
Fuelling, L. J., J. A. Adams, K. J. Badik, R. J. Bixby, C. L. Caprette, H. E. Caprette, W. B. Chiasson, C. L. Davies, D. T. DeColibus, K. I. Glascock, M. M. Hall, W. L. Perry, E. R. Schultz, D. A. Taylor, M. L. Vis & R. G. Verb, 2012. An unusual occurrence of Thorea hispida (Thore) Desvaux chantransia on rusty crayfish in West Central Ohio. Nova Hedwigia 94: 355–366.
Fullerton, A. H. & B. T. Watson, 2001. New distributional records for two nonindigenous and one native crayfish in North Carolina. Journal of the Elisha Mitchell Scientific Society 117: 66–70.
Garvey, J. E. & R. A. Stein, 1993. Evaluating how chela size influences the invasion potential of an introduced crayfish (Orconectes rusticus). The American Midland Naturalist 129: 172–181.
Garvey, J. E., R. A. Stein & H. M. Thomas, 1994. Assessing how fish predation and interspecific prey competition influence a crayfish assemblage. Ecology 75: 532–547.
Garvey, J. E., J. E. Rettig, R. A. Stein, D. M. Lodge & S. P. Klosiewski, 2003. Scale-dependent associations among fish predation, littoral habitat, and distributions of crayfish species. Ecology 84: 3339–3348.
Gaston, K. J., 2003. The Structure and Dynamics of Geographic Ranges. Oxford University Press, Oxford.
Gherardi, F. & W. H. Daniels, 2004. Agonism and shelter competition between invasive and indigenous crayfish species. Canadian Journal of Zoology 82: 1923–1932.
Girard, C. E., 1852. A revision of the North American Astaci, with observations on their habits and geographical distribution. Proceedings of the Academy of Natural Sciences of Philadelphia 6: 87–91.
Glon, M. G., E. R. Larson & K. L. Pangle, 2016a. Comparison of 13C and 15N discrimination factors and turnover rates between congeneric crayfish Orconectes rusticus and O. virilis (Decapoda, Cambaridae). Hydrobiologia 768: 51–61.
Glon, M. G., E. R. Larson & K. L. Pangle, 2016b. Connecting laboratory behavior to field function through stable isotope analysis. PeerJ 4: E1918.
Glon, M. G., E. R. Larson, L. S. Reisinger & K. L. Pangle, 2017. Invasive dreissenid mussels benefit invasive crayfish but not native crayfish in the Laurentian Great Lakes. Journal of Great Lakes Research 43: 289–297.
Goldsmith, T. H., 1978b. The spectral absorption of crayfish rhabdoms: pigment, photoproduct and pH sensitivity. Vision Research 18: 463–473.
Goldsmith, L. A., 1978a. The toxicity of parathion to Orconectes rusticus and Viviparus malleatus. Doctoral dissertation. University of Rhode Island, Kingston, RI.
Goldsmith, L. A. & G. P. Carlson, 1979. Divergent toxicity of parathion in two freshwater invertebrates, Orconectes rusticus and Viviparus malleatus. Journal of Environmental Science & Health, Part B 14: 579–588.
Govind, C. K., D. F. Mellon Jr. & M. M. Quigley, 1987. Muscle and muscle fiber type transformation in clawed crustaceans. American Zoologist 27: 1079–1098.
Green, N. S., B. A. Hazlett & S. Pruett-Jones, 2008. Attachment and shell integrity affect the vulnerability of zebra mussels (Dreissena polymorpha) to predation. Journal of Freshwater Ecology 23: 91–99.
Guiaşu, R. C., 2007. Conservation and diversity of the crayfishes of the genus Fallicambarus Hobbs, 1969 (Decapoda, Cambaridae), with an emphasis on the status of Fallicambarus fodiens (Cottle, 1863) in Canada. Crustaceana 80: 207–223.
Guiaşu, R. C., 2008. Specious claims? The Royal Ontario Museum Magazine 40: 26–33.
Guiaşu, R. C., 2009. Conservation, status, and diversity of the crayfishes of the genus Cambarus Erichson, 1846 (Decapoda, Cambaridae). Crustaceana 82: 721–742. https://doi.org/10.1163/156854009X407722.
Guiaşu, R. C., 2016. Non-native Species and Their Role in the Environment: The Need for a Broader Perspective. Brill Publishers, Leiden.
Guiaşu, R. C., 2021. Range expansion of the vulnerable crayfish Creaserinus fodiens (Cottle, 1863) (Decapoda, Cambaridae) in Ontario, Canada with added notes on the distribution, ecology and conservation status of this species in North America. Crustaceana. https://doi.org/10.1163/15685403-bja10104.
Guiaşu, R. C. & S. Guiaşu, 2003. Entropy in Ecology and Ethology. Nova Science Publishers Inc, New York.
Guiaşu, R. C. & C. W. Tindale, 2018. Logical fallacies and invasion biology. Biology & Philosophy 33: 34. https://doi.org/10.1007/s10539-018-9644-0.
Guiaşu, R. C., D. W. Barr & D. W. Dunham, 1996. Distribution and status of crayfishes of the genera Cambarus and Fallicambarus (Decapoda, Cambaridae) in Ontario, Canada. Journal of Crustacean Biology 16: 373–383.
Gunderson, J., 1995. Rusty Crayfish: A Nasty Invader. Minnesota Sea Grant, Duluth, Minnesota.
Hagen, H. A., 1870. Monograph of the North American Astacidae. Cambridge University Press, Cambridge, UK.
Hamr, P., 1998. Conservation Status of Canadian Freshwater Crayfishes. World Wildlife Fund and The Canadian Nature Federation, Toronto.
Hamr, P., 2002. Orconectes In Holdich, H. D. (ed), Biology of Freshwater Crayfish. Blackwell Science, Oxford, UK: 585-608.
Hamr, P., 2010. The biology, distribution and management of the introduced rusty crayfish, Orconectes rusticus (Girard), in Ontario, Canada. Freshwater Crayfish 17: 85–90.
Hansen, G. J. A., A. R. Ives, M. J. Vander Zanden & S. R. Carpenter, 2013c. Rapid transitions between invasive and native species. The Bulletin of the Ecological Society of America 94: 256–259.
Hansen, G. J. A., C. L. Hein, B. M. Roth, M. J. Vander Zanden, J. W. Gaeta, J. W. Latzka & S. R. Carpenter, 2013a. Food web consequences of long-term invasive crayfish control. Canadian Journal of Fisheries and Aquatic Sciences 70: 1109–1122.
Hansen, G. J. A., A. R. Ives, M. J. Vander Zanden & S. R. Carpenter, 2013b. Are rapid transitions between invasive and native species caused by alternative stable states, and does it matter? Ecology 94: 2207–2219.
Hansen, G. J. A., T. D. Tunney, L. A. Winslow & M. J. Vander Zanden, 2017. Whole-lake invasive crayfish removal and qualitative modeling reveal habitat-specific food web topology. Ecosphere 8: e01674.
Harlioğlu, M. M. & A. G. Harlioğlu, 2006. Threat of non-native crayfish introductions into Turkey: global lessons. Reviews in Fish Biology and Fisheries 16: 171–181.
Harris, J. A., 1903. An ecological catalogue of the crayfishes belonging to the genus Cambarus (Vol. 2, No. 3). University of Kansas, Lawrence, KS.
Hartzell, S. M., A. L. Pitt, S. Davis & S. T. Rier, 2018. Invasive rusty crayfish (Orconectes rusticus) are more active diurnally than a native congener (Orconectes limosus). Integrative and Comparative Biology 58: 334.
Hay, W. P., 1895. The crawfishes of the state of Indiana. Twentieth Annual Report of the Indiana Department of Geology and Nature 475-507.
Hay, W. P., 1899. Synopses of North-American Invertebrates. VI. The Astacidae of North America. The American Naturalist 33: 957–966.
Hay, W. P., 1902. Observations on the crustacean fauna of the region about Mammoth Cave, Kentucky. Proceedings of the United States National Museum.
Hayes, P. G., 1985. Crayfish puts pincer move on lakes. Milwaukee Journal 7.
Hayes, N. M., K. J. Butkas, J. D. Olden & M. J. Vander Zanden, 2009. Behavioural and growth differences between experienced and naïve populations of a native crayfish in the presence of invasive rusty crayfish. Freshwater Biology 54: 1876–1887.
Hazlett, B. A., 1985. Disturbance pheromones in the crayfish Orconectes virilis. Journal of Chemical Ecology 11: 1695–1711.
Hazlett, B. A., 1994. Crayfish feeding responses to zebra mussels depend on microorganisms and learning. Journal of Chemical Ecology 20: 2623–2630.
Hazlett, B. A., 2000. Information use by an invading species: do invaders respond more to alarm odors than native species? Biological Invasions 2: 289–294.
Hazlett, B. A., 2003. The effects of starvation on crayfish responses to alarm odor. Ethology 109: 587–592.
Hazlett, B. A., 2007. Conditioned reinforcement in the crayfish Orconectes rusticus. Behaviour 144: 847–859.
Hazlett, B. A. & D. R. Schoolmaster, 1998. Responses of cambarid crayfish to predator odor. Journal of Chemical Ecology 24: 1757–1770.
Hazlett, B. A., F. E. Anderson, L. A. Esman, L. A. Stafford & E. Munro, 1992. Interspecific behavioral ecology of the crayfish Orconectes rusticus. Journal of Freshwater Ecology 7: 69–76.
Hazlett, B. A., P. Acquistapace & F. Gherardi, 2002. Differences in memory capabilities in invasive and native crayfish. Journal of Crustacean Biology 22: 439–448.
Hein, C. L., B. M. Roth, A. R. Ives & M. J. Vander Zanden, 2006. Fish predation and trapping for rusty crayfish (Orconectes rusticus) control: a whole-lake experiment. Canadian Journal of Fisheries and Aquatic Sciences 63: 383–393.
Hein, C. L., M. J. Vander Zanden & J. J. Magnuson, 2007. Intensive trapping and increased fish predation cause massive population decline of an invasive crayfish. Freshwater Biology 52: 1134–1146.
Helgen, J. C., 1990. The distribution of crayfishes (Decapoda, Cambaridae) in Minnesota. Section of Fisheries, Minnesota Department of Natural Resources, Division of Fish and Wildlife.
Helms, B., Z. L. Loughman, B. L. Brown & J. Stoeckel, 2013. Recent advances in crayfish biology, ecology, and conservation. Freshwater Science 32: 1273–1275.
Henttonen, P., J. V. Huner & O. V. Lindqvist, 1994. Occurrence of Psorospermium sp. in several North American crayfish species, with comparative notes on Psorospermium haeckeli in the European crayfish Astacus astacus. Aquaculture 120: 209–218.
Hill, A. M. & D. M. Lodge, 1994. Diel changes in resource demand: competition and predation in species replacement among crayfishes. Ecology 75: 2118–2126.
Hill, A. M. & D. M. Lodge, 1999. Replacement of resident crayfishes by an exotic crayfish: the roles of competition and predation. Ecological Applications 9: 678–690.
Hill, A. M., D. M. Sinars & D. M. Lodge, 1993. Invasion of an occupied niche by the crayfish Orconectes rusticus: potential importance of growth and mortality. Oecologia 94: 303–306.
Hobbs, H. H., 1942. A generic revision of the crayfishes of the subfamily Cambarinae (Decapoda, Astacidae) with the description of a new genus and species. The American Midland Naturalist 28: 334–357.
Hobbs, H. H., 1948. Two new crayfishes of the genus Orconectes from Arkansas, with a key to the species of the hylas group (Decapoda, Astacidae). The American Midland Naturalist 39: 139–150.
Hobbs, H. H., 1967. The current status of the crayfishes listed by Girard (1852) in his “a revision of the North American Astaci…” (Decapoda, Astacidae). Crustaceana 12: 124–132.
Hobbs, H. H., 1972. Biota of freshwater ecosystems identification: manual 9 crayfishes (Astacidae) of North America. Environmental Protection Agency 1-173.
Hobbs, H. H., 1974. A checklist of the North and Middle American crayfishes (Decapoda: Astacidae and Cambaridae). Smithsonian Contributions to Zoology 166: 1–161.
Hobbs, H. H., 1989. An illustrated checklist of the American crayfishes (Decapoda: Astacidae, Cambaridae and Parastacidae). Smithsonian Contributions to Zoology 480: 1-236.
Hobbs, H. H. & J. P. Jass, 1988. The Crayfishes & Shrimp of Wisconsin (Cambaridae, Palaemonidae). Milwaukee Public Museum, Milwaukee.
Hobbs, H. H. & C. S. Shoup, 1942. On the crayfishes collected from the Big South Fork of the Cumberland River in Tennessee during the summer of 1938. The American Midland Naturalist 28: 634–643.
Hobbs, H. H., J. P. Jass & J. V. Huner, 1989. A review of global crayfish introductions with particular emphasis on two North American species (Decapoda, Cambaridae). Crustaceana 56: 299–316.
Holdich, D. M., 1999. The negative effects of established crayfish introductions. In Gherardi, F. & D. M. Holdich (eds), Crayfish in Europe as Alien Species: How to Make the Best of a Bad Situation. A.A. Balkema, Rotterdam: 31–48.
Holdich, D. M., 2002. Biology of Freshwater Crayfish. Blackwell Science, Oxford, UK.
Hollett, L., M. Berrill & L. Rowe, 1986. Variation in major ion concentration of Cambarus robustus and Orconectes rusticus following exposure to low pH. Canadian Journal of Fisheries and Aquatic Sciences 43: 2040–2044.
Houghton, D. C., J. J. Dimick & R. V. Frie, 1998. Probable displacement of riffle-dwelling invertebrates by the introduced rusty crayfish, Orconectes rusticus (Decapoda: Cambaridae) in a North-Central Wisconsin stream. The Great Lakes Entomologist 31: 2.
Huber, R., 2005. Amines and motivated behaviors: a simpler systems approach to complex behavioral phenomena. Journal of Comparative Physiology 191: 231–239.
Huber, R. & L. Schroeder, 2001. Fight strategies differ with size and allometric growth of claws in crayfish, Orconectes rusticus. Behaviour 138: 1437–1449.
Hubschman, J. H., 1967a. Effects of copper on the crayfish Orconectes rusticus (Girard). I. Acute toxicity. Crustaceana 12: 33–42.
Hubschman, J. H., 1967b. Effects of copper on the crayfish Orconectes rusticus (Girard). II. Mode of toxic action. Crustaceana 12: 141–160.
Huntsman, A. G., 1911. The freshwater malacostraca of Ontario. Contributions to Canadian Biology 1914: 145–163.
Huston, M. A., 1994. Biological Diversity: The Coexistence of Species On Changing Landscapes. Cambridge University Press, Cambridge, UK.
Ingold, J. L., L. A. Weigt & S. I. Guttman, 1988. Relationship between genetic variation in selected invertebrates and type of freshwater habitat. Biochemical Systematics and Ecology 16: 343–349.
James, J., F. M. Slater, I. P. Vaughan, K. A. Young & J. Cable, 2014. Comparing the ecological impacts of native and invasive crayfish: could native species’ translocation do more harm than good? Oecologia 178: 309–316.
Jansen, W., N. Geard, T. Mosindy, G. Olson & M. Turner, 2009. Relative abundance and habitat association of three crayfish (Orconectes virilis, O. rusticus, and O. immunis) near an invasion front of O. rusticus, and long-term changes in their distribution in Lake of the Woods. Canada. Aquatic Invasions 4: 627–649.
Jernelöv, A., 2017. The Long-Term Fate of Invasive Species: Aliens Forever or Integrated Immigrants with Time?. Springer, Cham, Switzerland.
Jezerinac, R. F., 1967. The distribution of crayfishes (Decapoda: Astacidae) of the Chagrin River, Northeastern Ohio. Doctoral dissertation. Ohio State University.
Jezerinac, R. F., 1974. A record of Orconectes virilis (Decapoda: Astacidae) from Ohio. Ohio Journal of Science 74: 263.
Jezerinac, R. F., 1982. Life-history notes and distributions of crayfishes (Decapoda: Cambaridae) from the Chagrin River Basin, Northeastern Ohio. The Ohio Journal of Science 82: 181–192.
Jezerinac, R. F., 1986. Endangered and threatened crayfishes (Decapoda: Cambaridae). The Ohio Journal of Science 86: 177–180.
Jezerinac, R. F., 1991. The distribution of crayfishes (Decapoda: Cambaridae) of the Licking River watershed, Eastcentral Ohio: 1972-1977. The Ohio Journal of Science 91: 108–111.
Jezerinac, R. F., G. W. Stocker & D. C. Tarter, 1995. The Crayfishes (Decapoda: Cambaridae) of West Virginia. Ohio Biological Survey, Columbus, OH.
Johnson, J. H. & C. C. Nack, 2010. Ontogenetic variation in food consumption of rusty crayfish (Orconectes rusticus) in a central New York stream. Journal of Freshwater Ecology 29: 59–64.
Johnson, P. T. J., J. D. Olden, C. T. Solomon & M. J. Vander Zanden, 2009. Interactions among invaders: community and ecosystem effects of multiple invasive species in an experimental aquatic system. Oecologia 159: 161–170.
Jonas, J. L., R. M. Claramunt, J. D. Fitzsimons, J. E. Marsden & B. J. Ellrott, 2005. Estimates of egg deposition and effects of lake trout (Salvelinus namaycush) egg predators in three regions of the Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 62: 2254–2264.
Jurcak, A. M., S. J. Gauthier & P. A. Moore, 2015. The effects of biodiesel and crude oil on the foraging behavior of rusty crayfish, Orconectes rusticus. Archives of Environmental Contamination and Toxicology 69: 557–565.
Kamran, M. & P. A. Moore, 2015. Comparative homing behaviors in two species of crayfish, Fallicambarus fodiens and Orconectes rusticus. Ethology 121: 775–784.
Kamran, M., M. E. Moore & P. A. Moore, 2018. Homing behavior following shelter displacement in two crayfishes, Creaserinus fodiens (Cottle, 1863) and Faxonius rusticus (Girard, 1852) (Decapoda: Astacidea: Cambaridae). Journal of Crustacean Biology 38: 531–538.
Keller, T. A., 1991. The effect of the branchiobdellid Annelid Cambarincola fallax on the growth rate and condition of the crayfish Orconectes rusticus. Journal of Freshwater Ecology 7: 165–171.
Keller, T. A. & B. A. Hazlett, 2010. Thermal preferences and distribution of Northern Michigan crayfishes. Northeastern Naturalist 17: 615–629.
Keller, R. P., K. Frang & D. M. Lodge, 2008. Preventing the spread of invasive species: economic benefits of intervention guided by ecological predictions. Conservation Biology 22: 80–88.
Keller, R. P., D. M. Lodge, M. A. Lewis & J. F. Shogren, 2009. Bioeconomics of Invasive Species: Integrating Ecology, Economics, Policy, and Management. Oxford University Press, Oxford.
Kenison, E. K., P. Y. Weldy & R. N. Williams, 2018. There must be something in the water: assessing the behavioral responses of rusty crayfish (Orconectes rusticus) to fish and amphibian predator kairomones. Journal of Ethology 36: 77–84.
Kershner, M. W. & D. M. Lodge, 1995. Effects of littoral habitat and fish predation on the distribution of an exotic crayfish, Orconectes rusticus. Journal of the North American Benthological Society 14: 414–422.
Khan, T. N., D. Lieb, M. McKinney & Z. Loughman, 2018. Reassessment of the crayfishes of the upper Ohio River Basin in Pennsylvania. Proceedings of the West Virginia Academy of Science 90.
Kiley, A. & C. F. Dineen, 1981. Crayfishes of Lake Wawasee. Proceedings of the Indiana Academy of Science 91: 211–212.
Kilian, J. V. & P. Ciccotto, 2011. First record of the invasive Orconectes rusticus (rusty crayfish) from the Potomac River, Maryland. Southeastern Naturalist 10: 553–557.
Kilian, J. V., A. J. Becker, S. A. Stranko, M. Ashton, R. Klauda, J. Gerber & M. Hurd, 2010. The status and distribution of Maryland crayfishes. Southeastern Naturalist 9: 11–33.
Klar, N. M. & P. H. Crowley, 2012. Shelter availability, occupancy, and residency in size-asymmetric contests between rusty crayfish, Orconectes rusticus. Ethology 118: 118–126.
Klocker, C. A. & D. L. Strayer, 2004. Interactions among an invasive crayfish (Orconectes rusticus), a native crayfish (Orconectes limosus), and native bivalves (Sphaeriidae and Unionidae). Northeastern Naturalist 11: 167–179.
Kozlowski, C., R. Voigt & P. A. Moore, 2003. Changes in odour intermittency influence the success and search behaviour during orientation in the crayfish (Orconectes rusticus). Marine and Freshwater Behaviour and Physiology 36: 97–110.
Kraatz, W. C., 1921. A preliminary general survey of the macrofauna of Mirror Lake on the Ohio State University campus. The Ohio Journal of Science 21: 137–182.
Krane, D. E., D. C. Sternberg & G. A. Burton, 1999. Randomly amplified polymorphic DNA profile-based measures of genetic diversity in crayfish correlated with environmental impacts. Environmental Toxicology and Chemistry 18: 504–508.
Kraus-Epley, K. E. & P. A. Moore, 2002. Bilateral and unilateral antennal lesions alter orientation abilities of the crayfish, Orconectes rusticus. Chemical Senses 27: 49–55.
Kraus-Epley, K. E., S. E. Lahman & P. A. Moore, 2015. Behaviorally-selective chemoreceptor lesions reveal two different chemically mediated orientation strategies in the rusty crayfish, Orconectes rusticus. Journal of Crustacean Biology 35: 753–762.
Kreps, T. A., 2009. Scaling up: Long-term, large-scale impacts of the invasion of lakes by the invasive rusty crayfish (Orconectes rusticus). Doctoral dissertation. University of Notre Dame, Notre Dame, IN.
Kreps, T. A., A. K. Baldridge & D. M. Lodge, 2012. The impact of an invasive predator (Orconectes rusticus) on freshwater snail communities: insights on habitat-specific effects from a multilake long-term study. Canadian Journal of Fisheries and Aquatic Sciences 69: 1164–1173.
Kreps, T. A., E. R. Larson & D. M. Lodge, 2016. Do invasive rusty crayfish (Orconectes rusticus) decouple littoral and pelagic energy flows in lake food webs? Freshwater Science 35: 103–113.
Kuhlmann, M. L., 2008. Do invading rusty crayfish interfere with reproduction in a native congener? Journal of Crustacean Biology 28: 461–465.
Kuhlmann, M. L. & P. D. Hazelton, 2007. Invasion of the upper Susquehanna River watershed by rusty crayfish (Orconectes rusticus). Northeastern Naturalist 14: 507–519.
Kuhlmann, M. L., S. M. Badylak & E. L. Carvin, 2007. Testing the differential predation hypothesis for the invasion of rusty crayfish in a stream community: laboratory and field experiments. Freshwater Biology 53: 113–128.
Lahman, S. E. & P. A. Moore, 2015. Olfactory sampling recovery following sublethal copper exposure in the rusty crayfish, Orconectes rusticus. Bulletin of Environmental Contamination and Toxicology 95: 441–446.
Lahman, S. E., K. R. Trent & P. A. Moore, 2015. Sublethal copper toxicity impairs chemical orientation in the crayfish, Orconectes rusticus. Ecotoxicology and Environmental Safety 113: 369–377.
Lang, J., 1977. Foreign crayfish taking over in some waters. Milwaukee Journal 30: 10.
Langlois, T. H., 1935. Notes on the habits of the crayfish, Cambarus rusticus Girard, in fish ponds in Ohio. Transactions of the American Fisheries Society 65: 189–193.
Langlois, T. H., 1937. Further observations on habits of the crayfish, Cambarus rusticus Girard. Transactions of the American Fisheries Society 66: 275–276.
Larson, E. R. & J. D. Olden, 2008. Do schools and golf courses represent emerging pathways for crayfish invasions? Aquatic Invasions 3: 465–468.
Larson, E. R. & J. D. Olden, 2010. Latent extinction and invasion risk of crayfishes in the southeastern United States. Conservation Biology 24: 1099–1110.
Larson, E. R. & J. D. Olden, 2011. The state of crayfish in the Pacific Northwest. Fisheries 36: 60–73.
Larson, E. R., M. A. Renshaw, C. A. Gantz, J. Umek, S. Chandra, D. M. Lodge & S. P. Egan, 2017. Environmental DNA (eDNA) detects the invasive crayfishes Orconectes rusticus and Pacifastacus leniusculus in large lakes of North America. Hydrobiologia 800: 173–185.
Latzka, A. W., J. T. Crawford, A. S. Kobblings, Y. Caldeira, E. Hilts & M. J. Vander Zanden, 2015. Representing calcification in distribution models for aquatic invasive species: surrogates perform as well as CaCO3 saturation state. Hydrobiologia 746: 197–208.
Lawton, S. M., 1979. A taxonomic and distributional study of crayfishes (Decapoda: Cambaridae) of West Virginia with diagnostic keys to the species of the genera. Master’s dissertation. Marshall University, Huntington, WV.
Layne Jr., J. R., M. L. Manis & D. L. Claussen, 1985. Seasonal variation in the time course of thermal acclimation in the crayfish Orconectes rusticus. Freshwater Invertebrate Biology 42: 98–104.
Layne Jr., J. R., D. L. Claussen & M. L. Manis, 1987. Effects of acclimation temperature, season, and time of day on the critical thermal maxima and minima of the crayfish Orconectes rusticus. Journal of Thermal Biology 12: 183–187.
Leon, M., M. J. Merner, A. A. Dreyer, A. Cooper, L. Scott, P. B. Berendzen, D. A. McCullough & E. C. Merten, 2016. Range expansion of the invasive rusty crayfish (Orconectes rusticus) (Decapoda: Astacoidea) in Northeastern Iowa (USA) Rivers. Journal of Crustacean Biology 36: 99–104.
Lewis, D. B., 2001. Trade-offs between growth and survival: responses of freshwater snails to predacious crayfish. Ecology 82: 758–762.
Lieb, D. A., R. F. Carline & H. M. Ingram, 2007. Status of native and invasive crayfish in ten National Park Service properties in Pennsylvania. Technical Report NPS/NER/NRTR - 2007/085. National Park Service. Philadelphia, PA.
Lieb, D. A., R. W. Bouchard & R. F. Carline, 2011. Crayfish fauna of southeastern Pennsylvania: distributions, ecology, and changes over the last century. Journal of Crustacean Biology 31: 166–178.
Linn, M. D. & P. A. Moore, 2014. The effects of Bt corn on rusty crayfish (Orconectes rusticus) growth and survival. Archives of Environmental Contamination and Toxicology 67: 436–443.
Lodge, D. M., 1993a. Species invasions and deletions: community effects and responses to climate and habitat change. In Kareiva, P. M., J. G. Kingsolver & R. B. Huey (eds), Biotic Interactions and Global Change. Sinauer Associates Inc., Sunderland, MA: 367–387.
Lodge, D. M., 1993b. Biological invasions: lessons for ecology. Trends in Ecology and Evolution 8: 133–137.
Lodge, D. M. & J. G. Lorman, 1987. Reductions in submersed macrophyte biomass and species richness by the crayfish Orconectes rusticus. Canadian Journal of Fisheries and Aquatic Sciences 44: 591–597.
Lodge, D. M., A. L. Beckel & J. J. Magnuson, 1985. Lake-bottom tyrant. Natural History 94: 32–37.
Lodge, D. M., T. K. Kratz & G. M. Capelli, 1986. Long-term dynamics of three crayfish species in Trout Lake, Wisconsin. Canadian Journal of Fisheries and Aquatic Sciences 43: 993–998.
Lodge, D. M., M. W. Kershner, J. E. Aloi & A. P. Covich, 1994. Effects of an omnivorous crayfish (Orconectes rusticus) on a freshwater littoral food web. Ecology 75: 1265–1281.
Lodge, D. M., R. A. Stein, K. M. Brown, A. P. Covich, C. Brönmark, J. E. Garvey & S. P. Klosiewskt, 1998. Predicting impact of freshwater exotic species on native biodiversity: challenges in spatial scaling. Australian Journal of Ecology 23: 53–67.
Lodge, D. M., C. A. Taylor, D. M. Holdich & J. Skurdal, 2000a. Nonindigenous crayfishes threaten North American freshwater biodiversity: lessons from Europe. Fisheries 25: 7–20.
Lodge, D. M., C. A. Taylor, D. M. Holdich & J. Skurdal, 2000b. Reducing impacts of exotic crayfish introductions: new policies needed. Fisheries 25: 21–23.
Lorman, J. G., 1975. Feeding and activity of the crayfish Orconectes rusticus in a northern Wisconsin lake. Master’s dissertation. University of Wisconsin-Madison, Madison, WI.
Lorman, J. G., 1980. Ecology of the crayfish Orconectes rusticus in Northern Wisconsin. Doctoral dissertation. University of Wisconsin-Madison, Madison, WI.
Lorman, J. G. & J. J. Magnuson, 1978. Role of crayfishes in aquatic ecosystems. Fisheries 3: 8.
Loughman, Z. J. & S. A. Welsh, 2010. Distribution and conservation standing of West Virginia Crayfishes. Southeastern Naturalist 9: 63–78.
Loughman, Z. J., T. P. Simon & S. A. Welsh, 2009. West Virginia Crayfishes (Decapoda: Cambaridae): observations on distribution, natural history, and conservation. Northeastern Naturalist 16: 225–239.
Luttenton, M. R., M. J. Horgan & D. M. Lodge, 1998. Effects of three Orconectes crayfishes on epilithic microalgae: a laboratory experiment. Crustaceana 71: 845–855.
Maezo, M. J., H. Fournier & B. E. Beisner, 2010. Potential and realized interactions between two aquatic invasive species: Eurasian watermilfoil (Myriophyllum spicatum) and rusty crayfish (Orconectes rusticus). Canadian Journal of Fisheries and Aquatic Sciences 67: 684–700.
Magnuson, J. J., G. M. Capelli, J. G. Lorman & R. A. Stein, 1975. Consideration of crayfish for macrophyte control. Proceedings of a Symposium on Water Quality Management through Biological Control, 66-74.
Magnuson, J. J., T. K. Kratz & B. J. Benson, 2006. Long-term Dynamics of Lakes in the Landscape: Long-term Ecological Research on North Temperate Lakes. Oxford University Press on Demand, Oxford.
Mangan, B. P. & M. D. Bilger, 2012. First record of phoresy between chironomid larvae and crayfish. The American Midland Naturalist 167: 410–416.
Marsden, J. E. & M. Hauser, 2009. Exotic species in Lake Champlain. Journal of Great Lakes Research 35: 250–265.
Martin, A. L. & P. A. Moore, 2007. Field observations of agonism in the crayfish, Orconectes rusticus: shelter use in a natural environment. Ethology 113: 1192–1201.
Martin, A. L. & P. A. Moore, 2008. The influence of dominance on shelter preference and eviction rates in the crayfish, Orconectes rusticus. Ethology 114: 351–360.
Martin, A. L. & P. A. Moore, 2010. The influence of reproductive state on the agonistic interactions between male and female crayfish (Orconectes rusticus). Behaviour 147: 1309–1325.
Martin, J. R., R. M. Pearce, J. R. Wohler, A. M. Monson & R. E. Bugbee, 1971. Analysis for ten minerals in the tissues of Orconectes rusticus (Girard) after exposure to increased concentrations of Ca, Cu, or Fe. Proceedings of the Pennsylvania Academy of Science 45: 180–187.
Mather, M. E. & R. A. Stein, 1993. Direct and indirect effects of fish predation on the replacement of a native crayfish by an invading congener. Canadian Journal of Fisheries and Aquatic Sciences 50: 1279–1288.
Maude, S. H., 1988. A new Ontario locality record for the crayfish Orconectes rusticus from the West Duffin Creek, Durham Regional Municipality. Canadian Field-Naturalist 102: 66–67.
Maude, S. H. & D. D. Williams, 1983. Behavior of crayfish in water currents: hydrodynamics of eight species with reference to their distribution patterns in southern Ontario. Canadian Journal of Fisheries and Aquatic Sciences 40: 68–77.
Mauro, N. A. & G. W. Moore, 1987. Effects of environmental pH on ammonia excretion, blood pH, and oxygen uptake in fresh water crustaceans. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 87: 1–3.
McBride, J., 1983. Meet the rusty crayfish. Wisconsin Sportsman 12: 42–46.
McCarthy, J. M., C. L. Hein, J. D. Olden & M. J. Vander Zanden, 2006. Coupling long-term studies with meta-analysis to investigate impacts of non-native crayfish on zoobenthic communities. Freshwater Biology 51: 224–235.
McFeeters, B. J., M. A. Xenopoulos, D. E. Spooner, N. D. Wagner & P. C. Frost, 2011. Intraspecific mass-scaling of field metabolic rates of a freshwater crayfish varies with stream land cover. Ecosphere 2: 1–10.
McMahon, B. R. & S. A. Stuart, 1989. The physiological problems of crayfish in acid waters. Acid Toxicity and Aquatic Animals 34: 171–199.
McMahon, B. R. & P. R. H. Wilkes, 1983. Emergence responses and aerial ventilation in normoxic and hypoxic crayfish Orconectes rusticus. Physiological Zoology 56: 133–141.
Minckley, W. L., 1963. The ecology of a spring stream: Doe Run, Meade County, Kentucky. Wildlife Monographs 11: 3–124.
Momot, W. T., 1984. Crayfish production: a reflection of community energetics. Journal of Crustacean Biology 4: 35–54.
Momot, W. T., 1991. Potential for exploitation of freshwater crayfish in coolwater systems: management guidelines and issues. Fisheries 16: 14–21.
Momot, W. T., 1992. Further range extensions of the crayfish Orconectes rusticus in the Lake Superior Basin of northwestern Ontario. Canadian Field-Naturalist 106: 397–399.
Momot, W. T., 1996. History of the range extension of Orconectes rusticus into northwestern Ontario and Lake Superior. Freshwater Crayfish 11: 61–72.
Momot, W. T., H. Gowing & P. D. Jones, 1978. The dynamics of crayfish and their role in ecosystems. American Midland Naturalist 99: 10–35.
Momot, W. T., C. Hartviksen & G. Morgan, 1988. A range extension for the crayfish Orconectes rusticus: Sibley Provincial Park, Northwestern Ontario. Canadian Field-Naturalist 102: 547–548.
Moore, P. A. & J. L. Grills, 1999. Chemical orientation to food by the crayfish Orconectes rusticus: influence of hydrodynamics. Animal Behaviour 58: 953–963.
Morehouse, R. L. & M. Tobler, 2013. Invasion of rusty crayfish, Orconectes rusticus, in the United States: niche shifts and potential future distribution. Journal of Crustacean Biology 33: 293–300.
Morgan, D. O. & B. R. McMahon, 1982. Acid tolerance and effects of sublethal acid exposure on iono-regulation and acid-base status in two crayfish Procambarus clarkii and Orconectes rusticus. Journal of Experimental Biology 97: 241–252.
Morrison, P. R., M. Pierce & F. A. Ryser, 1957. Food consumption and body weight in the masked and short-tail shrews. The American Midland Naturalist 57: 493–501.
Morse, J. W., A. K. Baldridge & A. W. Sargent, 2013. Invasive crayfish Orconectes rusticus (Decapoda, Cambaridae) is a more effective predator of substrate nesting fish eggs than native crayfish (O. virilis). Crustaceana 86: 387–402.
Mundahl, N. D., 1989. Seasonal and diel changes in thermal tolerance of the crayfish Orconectes rusticus, with evidence for behavioral thermoregulation. Journal of the North American Benthological Sciety 8: 173–179.
Mundahl, N. D. & M. J. Benton, 1990. Aspects of the thermal ecology of the rusty crayfish Orconectes rusticus (Girard). Oecologia 82: 210–216.
Nadelhoffer, K. J., A. J. Hogg & B. A. Hazlett, 2010. The Changing Environment of Northern Michigan: a Century of Science and Nature at the University of Michigan Biological Station. University of Michigan Press/Regional.
Nathaniel, T. H., J. Panksepp & R. Huber, 2009. Drug-seeking behavior in an invertebrate system: evidence of morphine-induced reward, extinction and reinstatement in crayfish. Behavioural Brain Research 197: 331–338.
Nathaniel, T. H., R. Huber & J. Panksepp, 2012. Repeated cocaine treatments induce distinct locomotor effects in crayfish. Brain Research Bulletin 87: 328–333.
Ngulo, E. M. & S. A. Grubbs, 2010. Relationships between crayfish abundance patterns and environmental variables across two spatial scales in a central Kentucky River Basin, USA. Journal of Freshwater Ecology 25: 285–295.
Nilsson, E., C. T. Solomon, K. A. Wilson, T. V. Willis, B. Larget & M. J. Vander Zanden, 2012. Effects of an invasive crayfish on trophic relationships in north-temperate lake food webs. Freshwater Biology 57: 10–23.
United States Geological Survey, 2012. Orconectes rusticus. USGS Nonindigenous Aquatic Species Database, Gainesville, FL. Available online at http://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=214. Revision Date: 3/10/2019.
Ogle, D. H. & L. Kret, 2008. Experimental evidence that captured rusty crayfish (Orconectes rusticus) exclude uncaptured rusty crayfish from entering traps. Journal of Freshwater Ecology 23: 123–129.
Olden, J. D., J. M. McCarthy, J. T. Maxted, W. W. Fetzer & M. J. Vander Zanden, 2006. The rapid spread of rusty crayfish (Orconectes rusticus) with observations on native crayfish declines in Wisconsin (U.S.A.) over the past 130 years. Biological Invasions 8: 1621–1628.
Olden, J. D., J. W. Adams & E. R. Larson, 2009. First record of Orconectes rusticus (Girard, 1852) (Decapoda, Cambaridae) west of the Great Continental Divide in North America. Crustaceana 82: 1347–1351.
Olden, J. D., M. J. Vander Zanden & P. T. Johnson, 2011. Assessing ecosystem vulnerability to invasive rusty crayfish (Orconectes rusticus). Ecological Applications 21: 2587–2599.
Olden, J. D. & M. Tamayo, 2014. Incentivizing the public to support invasive species management: Eurasian milfoil reduces lakefront property values. PloS one 9: e110458.
Olsen, T. M., D. M. Lodge, G. M. Capelli & R. J. Houlihan, 1991. Mechanisms of impact of an introduced crayfish (Orconectes rusticus) on littoral congeners, snails, and macrophytes. Canadian Journal of Fisheries and Aquatic Sciences 48: 1853–1861.
Orlova-Bienkowskaja, M. J. & M. G. Volkovitsh, 2018. Are native ranges of the most destructive invasive pests well known? A case study of the native range of the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). Biological Invasions 20: 1275–1286.
Ortmann, A. E., 1902. The geographical distribution of freshwater decapods and its bearing upon ancient geography. Proceedings of the American Philosophical Society 41: 267–400.
Ortmann, A. E., 1905. The mutual affinities of the species of the genus Cambarus, and their dispersal over the United States. Proceedings of the American Philosophical Society 44: 91–136.
Ortmann, A. E., 1906. The crawfishes of the state of Pennsylvania. Memoirs of the Carnegie Museum 2: 343–523.
Ortmann, A. E., 1913. The Alleghenian Divide, and its influence upon the freshwater fauna. Proceedings of the American Philosophical Society 52: 287–390.
Ortmann, A. E., 1931. Crawfishes of the southern Appalachians and the Cumberland Plateau. Annals of the Carnegie Museum 20: 61–160.
Osburn, R. C. & E. B. Williamson, 1898. The crayfishes of Ohio. 6th Annual Report of the Ohio Academy of Science 1-21.
Page, L. M., 1974. Aquatic Malacostraca recorded for Illinois, with notes on their distributions and habitats within the state. Transactions from the Illinois State Academy of Sciences 67: 89–104.
Page, C. H., 1975. Command fiber control of crayfish abdominal movement. Journal of Comparative Physiology 102: 65–76.
Page, L. M., 1985. The crayfishes and shrimps (Decapoda) of Illinois. Illinois Natural History Survey Bulletin 33: 1–448.
Page, L. M. & B. M. Burr, 1973. Distributional records for the crayfishes Cambarellus puer, C. shufeldtii, Procambarus gracilis, P. viaeviridis, Orconectes lancifer, O. bisectus, and O. rusticus. Transactions of the Kentucky Academy of Science 34: 51–52.
Panksepp, J. B. & R. Huber, 2002. Chronic alterations in serotonin function: dynamic neurochemical properties in agonistic behavior of the crayfish, Orconectes rusticus. Journal of Neurobiology 50: 276–290.
Panksepp, J. B. & R. Huber, 2004. Ethological analyses of crayfish behavior: a new invertebrate system for measuring the rewarding properties of psychostimulants. Behaviour Brain Research 153: 171–180.
Pearse, A. S., 1910. A preliminary list of the Crustacea of Michigan. Michigan Academy of Sciences 12: 68–76.
Pearse, A. S., M. T. Cook, A. D. Tinker & H. I. Smith, 1910. The Crawfishes of Michigan (Vol. 1). Wynkoop Hallenbeck Crawford co., state printers.
Pecor, K. W. & B. A. Hazlett, 2005. A test of temporal variation in risk and food stimuli on behavioral tradeoffs in the rusty crayfish, Orconectes rusticus: risk allocation and stimulus degradation. Ethology 112: 230–237.
Penn, G. H., 1950. Utilization of crawfishes by cold-blooded vertebrates in the Eastern United States. The American Midland Naturalist 44: 643–658.
Percival, D. & P. A. Moore, 2010. Shelter size influences self-assessment of size in crayfish, Orconectes rusticus: consequences for agonistic fights. Behaviour 147: 103–119.
Pereyra, P. J., 2020. Rethinking the native range concept. Conservation Biology 34: 373–377.
Pereyra, P. J., & R. C. Guiaşu, 2020. Debate over the importance and meaning of native range in invasion biology: reply to Courchamp et al. Conservation Biology 34: 1044–1046. https://doi.org/10.1111/cobi.13529.
Perry, W. L. & H. M. Jones, 2017. Effects of elevated water velocity on the invasive rusty crayfish (Orconectes rusticus Girard, 1852) in a laboratory mesocosm. Journal of Crustacean Biology 38: 13–22.
Perry, W. L., D. M. Lodge & G. A. Lamberti, 1997. Impact of crayfish predation on exotic zebra mussels and native invertebrates in a lake-outlet stream. Canadian Journal of Fisheries and Aquatic Sciences 54: 120–125.
Perry, W. L., D. M. Lodge & G. A. Lamberti, 2000. Crayfish (Orconectes rusticus) impacts on zebra mussel (Dreissena polymorpha) recruitment, other macroinvertebrates and algal biomass in a lake-outlet stream. The American Midland Naturalist 144: 308–317.
Perry, W. L., J. L. Feder, G. Dwyer & D. M. Lodge, 2001. Hybrid zone dynamics and species replacement between Orconectes crayfishes in a northern Wisconsin lake. Evolution 55: 1153–1166.
Perry, W. L., J. L. Feder & D. M. Lodge, 2002a. Implications of hybridization between introduced and resident Orconectes crayfishes. Conservation Biology 15: 1566–1666.
Perry, W. L., D. M. Lodge & J. L. Feder, 2002b. Importance of hybridization between indigenous and nonindigenous freshwater species: an overlooked threat to North American biodiversity. Systematic Biology 51: 255–275.
Perry, W. L., A. M. Jacks, D. Fiorenza, M. Young, R. Kuhnke & S. J. Jacquemin, 2013. Effects of water velocity on the size and shape of rusty crayfish, Orconectes rusticus. Freshwater Science 32: 1398–1409.
Peters, J. A. & D. M. Lodge, 2009. Invasive species policy at the regional level: a multiple weak links problem. Fisheries 34: 373–380.
Peters, J. A. & D. M. Lodge, 2013. Habitat, predation, and coexistence between invasive and native crayfishes: prioritizing lakes for invasion prevention. Biological Invasions 15: 2489–2502.
Peters, J. A., T. Kreps & D. M. Lodge, 2008. Assessing the impacts of rusty crayfish (Orconectes rusticus) on submergent macrophytes in a north-temperate U.S. lake using electric fences. The American Midland Naturalist 159: 287–298.
Peters, J. A., M. J. Cooper, S. M. Creque, M. S. Kornis, J. T. Maxted, W. L. Perry, F. W. Schueler, T. P. Simon, C. A. Taylor, R. F. Thoma, D. G. Uzarski & D. M. Lodge, 2014. Historical changes and current status of crayfish diversity and distribution in the Laurentian Great Lakes. Journal of Great Lakes Research 40: 35–46.
Phillips, G. S., 1980. The decapod crustaceans of Iowa. Proceedings of the Iowa Academy of Science 87: 81–95.
Phillips, G. S. & L. A. Reis, 1979. Distribution and ecology of Orconectes iowaensis Fitzpatrick and Orconectes rusticus (Girard) in Minnesota. Journal of the Minnesota Academy of Science 45: 18–19.
Phillips, I. D., R. D. Vinebrooke & M. A. Turner, 2009. Ecosystem consequences of potential range expansions of Orconectes virilis and Orconectes rusticus crayfish in Canada – a review. Environmental Reviews 17: 235–248.
Pilotto, F., G. Free, G. Crosa, F. Sena, M. Ghiani & A. C. Cardoso, 2008. The invasive crayfish Orconectes limosus in Lake Varese: estimating abundance and population size structure in the context of habitat and methodological constraints. Journal of Crustacean Biology 28: 633–640.
Pintor, L. M. & A. Sih, 2009. Differences in growth and foraging behavior of native and introduced populations of an invasive crayfish. Biological Invasions 11: 1895–1902.
Poly, W. J. & J. E. Wetzel, 2003. Distribution and taxonomy of three species of Orconectes (Decapoda: Cambaridae) in Illinois, U.S.A. Journal of Crustacean Biology 23: 380–390.
Prins, R., 1964. Attheyella carolinensis Chappuis (Copepoda: Harpacticoida) on freshwater crayfishes from Kentucky. Transactions of the American Microscopical Society 83: 370–371.
Prins, R., 1968. Comparative ecology of the crayfishes Orconectes rusticus rusticus and Cambarus tenebrosus in Doe Run, Meade County, Kentucky. Internationale Revue der gesamten Hydrobiologie und Hydrographie 53: 667–714.
Puth, L. M. & T. F. H. Allen, 2005. Potential corridors for the rusty crayfish, Orconectes rusticus, in Northern Wisconsin (USA) lakes: lessons for exotic invasions. Landscape Ecology 20: 567–577.
Rahel, F. J., 1988. Nest defense and aggressive interactions between a small benthic fish (the Johnny darter Etheostoma nigrum) and crayfish. Environmental Biology of Fishes 24: 301–306.
Rahel, F. J. & R. A. Stein, 1988. Complex predator-prey interactions and predator intimidation among crayfish, piscivorous fish, and small benthic fish. Oecologia 75: 94–98.
Reid, S. M., 2015. Optimizing sampling effort to detect rusty crayfish (Orconectes rusticus) in southern Ontario rivers. Management of Biological Invasions 6: 303–306.
Reid, S. M. & J. Devlin, 2014. Effectiveness of stream sampling methods in capturing non-native rusty crayfish (Orconectes rusticus) in Ontario. The Canadian Field-Naturalist 128: 111–118.
Reid, S. M. & J. J. Nocera, 2015. Composition of native crayfish assemblages in southern Ontario rivers affected by rusty crayfish (Orconectes rusticus Girard, 1852) invasions – implications for endangered queen snake recovery. Aquatic Invasions 10: 189–198.
Reisinger, L. S. & D. M. Lodge, 2016. Parasites alter freshwater communities in mesocosms by modifying invasive crayfish behavior. Ecology 97: 1497–1506.
Reisinger, L. S., I. Petersen, J. S. Hing, R. L. Davila & D. M. Lodge, 2015. Infection with a trematode parasite differentially alters competitive interactions and antipredator behaviour in native and invasive crayfish. Freshwater Biology 60: 1581–1595.
Reisinger, L. S., A. K. Elgin, K. M. Towle, D. J. Chan & D. M. Lodge, 2017. The influence of evolution and plasticity on the behavior of an invasive crayfish. Biological Invasions 19: 815–830.
Rhoades, R., 1944a. Further studies on distribution and taxonomy of Ohio crayfishes, and the description of a new subspecies. The Ohio Journal of Science 44: 95–99.
Rhoades, R., 1944b. The crayfishes of Kentucky, with notes on variation, distribution and descriptions of new species and subspecies. The American Midland Naturalist 31: 111–149.
Rhoades, R., 1962. Further studies on Ohio crayfishes: cases of sympatry of stream species in Southern Ohio. The Ohio Journal of Science 62: 27–33.
Ricciardi, A. & H. J. MacIsaac, 2008. The book that began invasion biology. Nature 452: 34.
Richmond, S. & D. C. Lazenby, 2005. The behavioural response of mayfly nymphs (Stenonema sp.) to chemical cues from crayfish (Orconectes rusticus). Hydrobiologia 560: 335–343.
Rietz, N. M., 1912. Ecological relations of the crawfishes of Illinois. Undergraduate dissertation. University of Illinois, Champaign, IL.
Rodríguez, G. & H. Suárez, 2001. Anthropogenic dispersal of decapod crustaceans in environments. Interciencia 26: 282–288.
Rosenburg, D. M., M. A. Turner, W. Jansen, T. Mosindy & D. A. Watkinson, 2010. Threats to Lake of the Woods and the Winnipeg River by the Rusty Crayfish (Orconectes rusticus), an Aquatic Invader. Ontario Ministry of Natural Resources, Northwest Science and Information Technical Workshop Report. TWR-005, 54 pp.
Rosenthal, S. K., S. S. Stevens & D. M. Lodge, 2006. Whole-lake effects of invasive crayfish (Orconectes spp.) and the potential for restoration. Canadian Journal of Fisheries and Aquatic Sciences 63: 1276–1285.
Rossner, K. L. & R. G. Sherman, 1978. Invaginated membrane in crustacean tonic muscle fibers: estimates of membrane capacitance. American Journal of Physiology-Cell Physiology 235: 220–226.
Roth, B. M. & J. F. Kitchell, 2005. The role of size-selective predation in the displacement of Orconectes crayfishes following rusty crayfish invasion. Crustaceana 78: 297–310.
Roth, B. M., C. L. Hein & M. J. Vander Zanden, 2006. Using bioenergetics and stable isotopes to assess the trophic role of rusty crayfish (Orconectes rusticus) in lake littoral zones. Canadian Journal of Fisheries and Aquatic Sciences 63: 335–344.
Roth, B. M., J. C. Tetzlaff, M. L. Alexander & J. F. Kitchell, 2007. Reciprocal relationships between exotic rusty crayfish, macrophytes, and Lepomis Species in Northern Wisconsin Lakes. Ecosystems 10: 75–86.
Roth, B. M., N. E. Mandrak, T. R. Hrabik, G. G. Sass & J. Peters, 2012. Fishes and decapod crustaceans of the Great Lakes basin. Great Lakes Policy and Management 2: 105–135.
Rutherford, P. L., D. W. Dunham & V. Allison, 1995. Winning agonistic encounters by male crayfish Orconectes rusticus (Girard) (Decapoda, Cambaridae): chela size matters but chela symmetry does not. Crustaceana 68: 526–529.
Rutherford, P. L., D. W. Dunham & V. Allison, 1996. Antennule use and agonistic success in the crayfish Orconectes rusticus (Girard, 1852) (Decapoda, Cambaridae). Crustaceana 69: 117–122.
Sargent, L. W. & D. M. Lodge, 2014. Evolution of invasive traits in nonindigenous species: increased survival and faster growth in invasive populations of rusty crayfish (Orconectes rusticus). Evolutionary Applications 7: 949–961.
Sargent, L. W., A. K. Baldridge, M. Vega-Ross, K. M. Towle & D. M. Lodge, 2014. A trematode parasite alters growth, feeding behavior, and demographic success of invasive rusty crayfish (Orconectes rusticus). Oecologia 175: 947–958.
Schlaepfer, M. A., D. F. Sax & J. D. Olden, 2011. The potential conservation value of non-native species. Conservation Biology 25: 428–437.
Schneider, R. A. Z., R. Huber & P. A. Moore, 2001. Individual and status recognition in the crayfish, Orconectes rusticus: the effects of urine release on fight dynamics. Behaviour 138: 137–153.
Sharma, M. L., 1966. Studies on the changes in the pattern of nitrogenous excretion of Orconectes rusticus under osmotic stress. Comparative Biochemistry and Physiology 19: 681–690.
Sharma, M. L., 1968. Studies on the sources and mechanism of increased urea production by Orconectes rusticus under osmotic stress. Comparative Biochemistry and Physiology 24: 55–60.
Sharma, M. L., 1969. Trigger mechanism of increased urea production by the crayfish, Orconectes rusticus under osmotic stress. Comparative Biochemistry and Physiology 30: 309–321.
Sharma, M. L. & M. C. Neveu, 1971. Studies on the role of ornithine cycle and purine catabolism in urea biosynthesis in the fresh-water crayfish, Orconectes rusticus. Comparative Biochemistry and Physiology 40: 863–870.
Simon, T. P., 2001. Checklist of the crayfish and freshwater shrimp (Decapoda) of Indiana. Proceedings of the Indiana Academy of Science 110: 104–110.
Simon, T. P., 2011. Conservation status of North American freshwater crayfish (Decapoda: Cambaridae) from the southern United States. Proceedings of the Indiana Academy of Science 120: 71–95.
Simon, J. L. & P. A. Moore, 2007. Male–female communication in the crayfish Orconectes rusticus: the use of urinary signals in reproductive and non-reproductive pairings. Ethology 113: 740–754.
Simon, T. P. & R. F. Thoma, 2006. Conservation of imperiled crayfish—Orconectes (Faxonius) indianensis Hay (Decapoda: Cambaridae). Journal of Crustacean Biology 26: 436–440.
Simon, T. P., A. E. Timm & C. C. Morris, 2005a. Orconectes (Procericambarus) theaphionensis (Decapoda: Cambaridae), the sinkhole crayfish, a new species of crayfish from southcentral Indiana. Proceedings of the Indiana Academy of Science 114: 43–54.
Simon, T. P., M. Weisheit, E. Seabrook, L. Freeman, S. Johnson, L. Englum, K. W. Jorck, M. Abernathy & T. P. Simon IV, 2005b. Notes on Indiana crayfish (Decapoda: Cambaridae) with comments on distribution, taxonomy, life history, and habitat. Proceedings of the Indiana Academy of Science 114: 55–61.
Smily Jr., P. C. & E. D. Dibble, 2000. Microhabitat use of an introduced crayfish (Orconectes rusticus) in Long Lake, Wisconsin. Journal of Freshwater Ecology 15: 115–123.
Smith, D. G., 1981. Evidence for hybridization between two crayfish species (Decapoda: Cambaridae: Orconectes) with a comment on the phenomenon in Cambarid crayfish. American Midland Naturalist 105: 405–407.
Smith, M. R. & D. W. Dunham, 1996. Antennae mediate agonistic physical contact in the crayfish Orconectes rusticus (Girard, 1852) (Decapoda, Cambaridae). Crustaceana 69: 668–674.
Snedden, W. A., 1988. Reproduction in the temperate crayfish, Orconectes rusticus: intermale aggression, copulatory behaviour, and sperm competition. Master’s dissertation. Brock University, St. Catherines, ON.
Snedden, W. A., 1990. Determinants of male mating success in the temperate crayfish Orconectes rusticus: chela size and sperm competition. Behaviour 115: 100–113.
Sohn, L., R. J. Brodie, G. Couldwell, E. Demmons & J. Sturve, 2018. Exposure to a nicotinoid pesticide reduces defensive behaviors in a non-target organism, the rusty crayfish Orconectes rusticus. Ecotoxicology 27: 900–907.
Solomon, C. T., J. D. Olden, P. T. J. Johnson, R. T. Dillon & M. J. Vander Zanden, 2010. Distribution and community-level effects of the Chinese mystery snail (Bellamya chinensis) in northern Wisconsin lakes. Biological Invasions 12: 1591–1605.
Somers, K. M. & R. A. Reid, 2010. The Dorset Environmental Science Centre crayfish sampling protocol. Threats to Lake of the Woods and the Winnipeg River by the rusty crayfish 14-24.
Sorenson, K., S. Bollens & T. Counihan, 2012. Rapid range expansion of rusty crayfish Orconectes rusticus (Girard, 1852) in the John Day River, Oregon, USA. Aquatic Invasions 7: 291–294.
Spaziani, E., T. C. Jegla, W. L. Wang, J. A. Booth, S. M. Connolly, C. C. Conrad, M. J. Dewall, C. M. Sarno, D. K. Stone & R. Montgomery, 2001. Further studies on signaling pathways for ecdysteroidogenesis in crustacean Y-organs. American Zoologist 41: 418–429.
Spoor, W. A., 1955. Loss and gain of heat-tolerance of the crayfish. The Biological Bulletin 108: 77–87.
St. John, F. L., 1982. Crayfish and bivalve distribution in a valley in Southwestern Ohio. The Ohio Journal of Science 82: 242–246.
St. John, F. L., 1988. Distribution and status of Orconectes (Rhoadesius) sloanii (Bundy) (Crustacea: Decapoda: Cambaridae). The Ohio Journal of Science 88: 202–204.
St. John, F. L., 1991. Brief Note: changes in mixed populations of Orconectes (R.) sloanii and O. (P.) rusticus (Crustacea: Decapoda: Cambaridae) in Southwestern Ohio. The Ohio Journal of Science 91: 172–173.
Steele, M., 1902. The crayfish of Missouri. University of Cincinnati Bulletin 10: 1–54.
Steele, C. W., S. Stricker-Shaw & D. H. Taylor, 1992. Attraction of crayfishes Procambarus clarkii, Orconectes rusticus and Cambarus bartoni to a feeding stimulant and its suppression by a blend of metals. Environmental Toxicology and Chemistry 11: 1323–1329.
Steele, C., C. Skinner, P. Alberstadt & J. Antonelli, 1997. Importance of adequate shelters for crayfishes maintained in aquaria. Aquarium Sciences and Conservation 1: 189–192.
Steele, C., C. Skinner, C. Steele, P. Alberstadt & C. Mathewson, 1999. Organization of chemically activated food search behavior in Procambarus clarkii Girard and Orconectes rusticus Girard crayfishes. The Biological Bulletin 196: 295-302.
Stephens, G. C., 1952. The control of cement gland development in the crayfish, Cambarus. The Biological Bulletin 103: 242–258.
Stewart, T. W., J. G. Milner & R. L. Lowe, 1998. An experimental analysis of crayfish (Orconectes rusticus) effects on a Dreissena-dominated benthic macroinvertebrate community in western Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 55: 1043–1050.
Stocker, A. M. & R. Huber, 2001. Fighting strategies in crayfish Orconectes rusticus (Decapoda, Cambaridae) differ with hunger state and the presence of food cues. Ethology 107: 727–736.
Strayer, D. L., 2010. Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshwater Biology 55: 152–174.
Sullivan, J. M. & B. S. Beltz, 2001. Neural pathways connecting the deutocerebrum and lateral protocerebrum in the brains of decapod crustaceans. Journal of Comparative Neurology 441: 9–22.
Symstad, A. J., F. S. Chapin, D. H. Wall, K. L. Gross, L. F. Huenneke, G. G. Mittlebach, D. P. C. Peters & D. Tilman, 2003. Long-term and large-scale perspectives on the relationship between biodiversity and ecosystem functioning. Bioscience 53: 89–98.
Szela, K. & W. L. Perry, 2013. Laboratory competition hierarchies between potentially invasive rusty crayfish (Orconectes rusticus) and native crayfishes of conservation concern. The American Midland Naturalist 169: 345–354.
Taylor, C. A., 2000. Systematic studies of the Orconectes juvenilis complex (Decapoda: Cambaridae), with descriptions of two new species. Journal of Crustacean Biology 20: 132–152.
Taylor, C. A. & M. Redmer, 1996. Dispersal of the crayfish Orconectes rusticus in Illinois, with notes on species displacement and habitat preference. Journal of Crustacean Biology 16: 547–551.
Taylor, C. A. & D. J. Soucek, 2010. Re-examining the importance of fish in the diets of stream-dwelling crayfishes: implications for food web analyses and conservation. The American Midland Naturalist 163: 280–294.
Taylor, C. A., M. L. Warren Jr., J. F. Fitzpatrick Jr., H. H. Hobbs, R. F. Jezerinac, W. L. Pflieger & H. W. Robinson, 1996. Conservation status of crayfishes of the United States and Canada. Fisheries 21: 25–38.
Taylor, C. A., G. A. Schuster, J. E. Cooper, R. J. DiStefano, A. G. Eversole, P. Hamr, H. H. Hobbs, H. W. Robison, C. E. Skelton & R. F. Thoma, 2007. A reassessment of the conservation status of crayfishes of the United States and Canada after 10 + years of increased awareness. Fisheries 32: 372–389.
Tetzlaff, J. C., B. M. Roth, B. C. Weidel & J. F. Kitchell, 2011. Predation by native sunfishes (Centrarchidae) on the invasive crayfish Orconectes rusticus in four northern Wisconsin lakes. Ecology of Freshwater Fish 20: 133–143.
Thoma, R. F. & R. F. Jezerinac, 2000. Ohio Crayfish and Shrimp Atlas. College of Biological Sciences, Ohio State University, Columbus, OH.
Thomas, C. L. & C. A. Taylor, 2013. Scavenger or predator? Examining a potential predator–prey relationship between crayfish and benthic fish in stream food webs. Freshwater Science 32: 1309–1317.
Thomaz, S. M., K. E. Kovalenko, J. E. Havel & L. B. Kats, 2015. Aquatic invasive species: general trends in the literature and introduction to the special issue. Hydrobiologia 746: 1–12.
Tierney, A. J., 2009. Differential growth in body and chela size following molts to Form I and Form II in the crayfish, Orconectes rusticus (Decapoda, Cambaridae). Crustaceana 82: 1527–1533.
Tierney, A. J. & K. Andrews, 2013. Spatial behavior in male and female crayfish (Orconectes rusticus): learning strategies and memory duration. Animal Cognition 16: 23–34.
Tierney, A. J. & J. Atema, 1988. Behavioral responses of crayfish (Orconectes virilis and Orconectes rusticus) to chemical feeding stimulants membrane capacitance. Journal of Chemical Ecology 14: 123–133.
Tierney, A. J. & D. W. Dunham, 1984. Behavioral mechanisms of reproductive isolation in crayfishes of the genus Orconectes. The American Midland Naturalist 111: 304–310.
Tierney, A. J. & J. Lee, 2011. Spatial learning in a T-maze by the crayfish Orconectes rusticus. Journal of Comparative Psychology 125: 31–39.
Tierney, A. J., M. S. Godleski & M. S. Massanari, 2000. Comparative analysis of agonistic behavior in four crayfish species. Journal of Crustacean Biology 20: 54–66.
Tierney, A. J., T. Kim & R. Abrams, 2003. Dopamine in crayfish and other crustaceans: distribution in the central nervous system and physiological functions. Microscopy Research and Technique 60: 325–335.
Tierney, A. J., M. A. Greenlaw, K. Dams-O’Connor, S. D. Aig & A. M. Perna, 2004. Behavioral effects of serotonin and serotonin agonists in two crayfish species, Procambarus clarkii and Orconectes rusticus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 139: 495–502.
Tierney, A. J., C. Gunarathe, K. Jennison, V. Monroy & L. Donnelly, 2008. Behavioral correlates of alternate male forms (Form I and Form II) in the crayfish Orconectes rusticus. Journal of Crustacean Biology 28: 596–600.
Tierney, A. J., K. N. Hanzlik, R. M. Hathaway, C. Powers & M. Roy, 2016. Effects of fluoxetine on growth and behavior in the crayfish Orconectes rusticus. Marine and Freshwater Behaviour and Physiology 49: 133–145.
Turner, C. L., 1926. The Crayfishes of Ohio. Ohio State University, Columbus, OH.
Twardochleb, L. A., J. D. Olden & E. R. Larson, 2013. A global meta-analysis of the ecological impacts of nonnative crayfish. Freshwater Science 32: 1367–1382.
Underwood, L. M., 1886. List of described species of fresh water Crustacea from America, north of Mexico. Illinois Natural History Survey Bulletin 2: 321–386.
Van den Brink, A. M. & C. L. McLay, 2009. Use of the sterile male technique to investigate sperm competition, storage, and use in a pill box crab, Halicarcinus cookii (Brachyura: Hymenosomatidae). Journal of Crustacean Biology 29: 62–69.
Vevea, D. & K. D. Hall, 1984. The effects of water temperature on molting and egg production following eyestalk ablation in two species of crayfish, Orconectes rusticus and Orconectes propinquus. Bios 55: 135–143.
Vollmer, K. L. & B. G. Gall, 2014. Complex predator–prey interactions between the rusty crayfish (Orconectes rusticus) and invertebrate and vertebrate prey within their native range. Journal of Freshwater Ecology 29: 267–277.
Von Humboldt, A., 1805. Essai sur la Géographie des Plantes. Levrault Schoell, Paris.
Wallace, A. R., 1876. The Geographical Distribution of Animals. Macmillan, London.
Waywell, E. B. & S. Corey, 1970. The presence of pteridines in the hypodermis as a taxonomic tool in crayfish. Canadian Journal of Zoology 48: 1462–1464.
Weisbord, C. D., D. T. Callaghan & D. T. Pyle, 2011. Associative learning in male rusty crayfish (Orconectes rusticus): conditioned behavioural response to an egg cue from walleye (Sander vitreus). Canadian Journal of Zoology 90: 85–92.
Wetzel, J. E., W. J. Poly & J. W. Fetzner Jr., 2004. Morphological and genetic comparisons of golden crayfish, Orconectes luteus, and rusty crayfish, O. rusticus, with range corrections in Iowa and Minnesota. Journal of Crustacean Biology 24: 603–617.
Whitledge, G. W. & C. F. Rabeni, 2002. Maximum daily consumption and respiration rates at four temperatures for five species of crayfish from Missouri, U.S.A. (Decapoda, Orconectes spp.). Crustaceana 75: 1119–1132.
Wilkes, P. R. H. & B. R. McMahon, 1982a. Effect of maintained hypoxic exposure on the crayfish Orconectes rusticus: I. Ventilatory, acid-base and cardiovascular adjustments. Journal of Experimental Biology 98: 119–137.
Wilkes, P. R. H. & B. R. McMahon, 1982b. Effect of maintained hypoxic exposure on the crayfish Orconectes rusticus: II. Modulation of haemocyanin oxygen affinity. Journal of Experimental Biology 98: 139–149.
Williams, P. R., 1967. Metacercariae of Prosthodendrium naviculum Macy, 1936 (Trematoda: Lecithodendriidae) from the crayfish, Orconectes rusticus (Girard). Proceedings of the Pennsylvania Academy of Science 41: 38–41.
Williams, A. B. & A. B. Leonard, 1952. The crayfishes of Kansas. Kansas University Science Bulletin 34: 960–1012.
Williamson, E. B., 1898. Notes on Ohio Astacidae. Annual Report of the Ohio Academy of Science 7: 47–48.
Williamson, E. B., 1901. The crayfish of Allegheny County, Pennsylvania. Annals of the Carnegie Museum 1: 8–13.
Williamson, E. B., 1907. Notes on the crayfish of Wells County, Indiana: with description of a new species. Report of State Geologist 749-763.
Willman, E. J., A. M. Hill & D. M. Lodge, 1994. Response of three crayfish congeners (Orconectes spp.) to odors of fish carrion and live predatory fish. The American Midland Naturalist 132: 44–51.
Wilson, K. A., 2002. Impacts of the invasive rusty crayfish (Orconectes rusticus) in northern Wisconsin lakes. Dissertation Abstracts International Part B: Science and Engineering 63: 1662.
Wilson, K. A., J. J. Magnuson, D. M. Lodge, A. M. Hill, T. K. Kratz, W. L. Perry & T. V. Willis, 2004. A long-term rusty crayfish (Orconectes rusticus) invasion: dispersal patterns and community change in a north temperate lake. Canadian Journal of Fisheries and Aquatic Sciences 61: 2255–2266.
Wofford, S. J., P. M. LaPlante & P. A. Moore, 2017. Information depends on context: behavioural response to chemical signals depends on sex and size in crayfish contests. Behaviour 154: 287–312.
Wolfe, D. A., 1964. The composition of crayfish carotenoids and the fatty acid composition of crayfish lipids including carotenoid esters. Doctoral dissertation. Ohio State University, Columbus, OH.
Wolfe, D. A. & D. G. Cornwell, 1965. Composition and tissue distribution of carotenoids in crayfish. Comparative Biochemistry and Physiology 16: 205–213.
Wolfe, D. A., P. V. Rao & D. G. Cornwell, 1965. Studies on the fatty acid composition of crayfish lipids. Journal of the American Oil Chemists’ Society 42: 633–637.
Wolf, M. C. & P. A. Moore, 2002. Effects of the herbicide metolachlor on the perception of chemical stimuli by Orconectes rusticus. Journal of the North American Benthological Society 21: 457–467.
Wood, C. M. & M. S. Rogano, 1986. Physiological responses to acid stress in crayfish (Orconectes): haemolymph ions, acid–base status, and exchanges with the environment. Canadian Journal of Fisheries and Aquatic Sciences 43: 1017–1026.
Yazicioglu, B., P. Hamr, P. Kozàk, A. Kouba & H. Niksirat, 2016. Fine structure of the spermatozoon in three species of Cambaridae (Arthropoda: Crustacea: Decapoda) Cambarus robustus, Orconectes propinquus and Orconectes rusticus: a comparative biometrical study. PeerJ 4: e2363.
Zuber, S. T., K. Muller, R. H. Laushman & A. J. Roles, 2012. Hybridization between an invasive and a native species of the crayfish genus Orconectes in North-Central Ohio. Journal of Crustacean Biology 32: 962–971.
Zulandt, T., R. A. Zulandt-Schneider & P. A. Moore, 2008. Observing agonistic interactions alters subsequent fighting dynamics in the crayfish, Orconectes rusticus. Animal Behaviour 75: 13–20.
Author information
Authors and Affiliations
Contributions
Radu Guiaşu initiated and designed the study and summarized and analyzed the data in Table 1 and some of the data in Table 2. Material preparation, data collection, and analysis were performed by Mark Labib and Radu Guiaşu. The final version of the manuscript was written mainly by Radu Guiaşu with contributions from Mark Labib. Both authors offered input on previous versions of the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Additional information
Handling editor: Lee B. Kats
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guiaşu, R.C., Labib, M. The unreliable concept of native range as applied to the distribution of the rusty crayfish (Faxonius rusticus) in North America. Hydrobiologia 848, 1177–1205 (2021). https://doi.org/10.1007/s10750-021-04523-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04523-y