Abstract
Following their invasion, non-indigenous fish can potentially exclude native fishes from their original niches through competition, or can partition in their resource use with native species to facilitate co-existence. Here, using stable isotope analysis, the trophic interactions of invasive European barbel Barbus barbus and other fishes were tested in an invaded river of relatively low fish species diversity and where no other Barbus species were present. Testing was over three distinct life stages: age 0 + (< 38 mm), juveniles (86–231 mm) and adults (> 386 mm). There were strong patterns of isotopic niche partitioning between the juvenile fishes, with some inter-specific niche differences also apparent in 0 + fishes. For adult B. barbus and chub Squalius cephalus, however, niche convergence was evident. Within the B. barbus population, the niches of the adult fish differed significantly from the 0+ and juvenile fish, indicating considerable dietary changes with development. These results suggested that niche partitioning at the most abundant life stages were facilitating the co-existence of invasive B. barbus with other fishes in the community, with this most likely driven by inter-specific differences in functional morphology and habitat use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasions of non-indigenous fishes can result in adverse impacts in native fish communities, including competitive displacement and exclusion (Gozlan et al., 2010). Understanding how an invasive fish can impact native species requires knowledge on their trophic interactions, such as whether they share prey resources, resulting in niche convergence, or exploit different resources, resulting in niche partitioning (Cucherousset et al., 2012; Tran et al., 2015; Copp et al., 2017). Quantifying the feeding relationships of introduced and native fishes is thus important for understanding their ecological risks to the native communities (Cucherousset & Olden, 2011) and facilitates assessment of the ecological impacts that might develop (Gozlan et al., 2010; Tran et al., 2015; Copp et al., 2017).
European barbel Barbus barbus (Linneaus, 1758) of the Cyprinidae family is now invasive in many European rivers outside of its native range, especially rivers in Italy and Western Britain (Britton & Pegg, 2011). Attaining lengths to approximately 800 mm and weights in excess of 8 kg (Amat-Trigo et al., 2017), they are generally valued for sport angling, with this the primary driver of introductions (Britton & Pegg, 2011). A highly vagile species, they can disperse relatively quickly through river systems (Hunt & Jones, 1974), often leading to rapid colonisation (Carosi et al., 2017). In invaded rivers where they are sympatric with endemic Barbus fishes, such as in Italian rivers (e.g. the Tiber basin), long-term data suggest populations of endemic Barbus tyberinus Bonaparte, 1839 are being displaced by B. barbus, with the mechanism suggested to involve asymmetric competition between the fishes (Carosi et al., 2017). This displacement is in addition to genetic impacts caused by introgression that results in a loss of genetic integrity in the endemic Barbus fishes (Meraner et al., 2013; Zaccara et al., 2014). Elsewhere in Europe, invasive B. barbus populations are often present in communities where other Barbus fishes are absent. Thus, whilst they are sympatric with indigenous cyprinid fishes such as chub Squalius cephalus (Linnaeus, 1758), they have lower functionally similarity with these fishes than with congeners. Consequently, the strength of their interactions might be less intense and their invasion might be less likely to incur negative ecological impacts.
Examples of systems invaded by B. barbus and where endemic Barbus fishes are absent are rivers in Western England. In Britain, B. barbus is only indigenous to eastern flowing rivers in England due to their previous connections with mainland Europe at the end of the last glacial period (Wheeler & Jordan, 1990). Research on these indigenous B. barbus suggests many of these populations are imperilled due to losses of habitat and river connectivity (Bašić et al., 2017). Consequently, enhancement stocking often supports these populations, with hatchery-reared individuals released at lengths between 120 and 250 mm and age 1+ and 2+ years (Britton et al., 2004; Antognazza et al., 2016). Studies on the trophic interactions of these stocked fish suggest substantial partitioning in their trophic niches with S. cephalus, the species that has the most similar functional traits and body sizes as B. barbus in these rivers (Bašic & Britton, 2016).
In their invasive range in Western England, populations tend to be more successful than many indigenous populations, with populations being relatively abundant and widespread through basins such as the River Severn (Amat-Trigo et al., 2017). Although knowledge of invasive B. barbus trophic interactions with indigenous fishes is limited in these rivers, both Bašić et al. (2015) and Gutmann Roberts et al. (2017) revealed that in rivers in both their native and invasive ranges, angling baits based on marine-derived nutrients can provide a strong trophic subsidy. This results in some individual B. barbus and S. cephalus (generally > 400 mm) specialising on this allochthonous resource. However, in rivers where angling is less intense and so where this subsidy is lower, and in body sizes that rarely consume these baits (< 400 mm), there remains a distinct knowledge gap on the trophic relationships of invasive B. barbus with other species. Moreover, there is also minimal knowledge on how their diet and trophic niche sizes change with increasing body size, and in relation to these changes in the indigenous fishes. This is despite the diet of fish usually being gape limited, where gape size is a function of body length (e.g. Persson et al., 1996), suggesting considerable dietary shifts will occur with increasing body length. Data on the inter- and intra-specific trophic relationships of B. barbus are also missing in their invasive range more generally, where competitive interactions between invasive and endemic Barbus fishes have, to date, been inferred from relative body condition data (e.g. Carosi et al., 2017).
The aim of this study was to quantify the trophic interactions of a population of invasive B. barbus with other fishes in a river where no other Barbus fishes were present. The focus was on determining the extent of trophic niche sharing within and between species, and how this altered across a range of life stages (as inferred from body sizes). The River Teme, western England, was the study river, where non-indigenous B. barbus have been present since the 1970s (Antognazza et al., 2016). The objective was to determine the trophic niche sizes and overlaps between invasive B. barbus and native fishes at three different life stages: age 0+ (young-of-the-year), juveniles and adults. It was hypothesised that (1) due to the consistent patterns of inter-specific trophic partitioning between B. barbus and native cyprinid fishes in their indigenous range (Bašic & Britton, 2016), these patterns of inter-specific partitioning are present in their non-indigenous, invasive range; and (2) within the fishes, there were significant shifts in the position of the trophic niches across the three life stages, with populations having a relatively large niche comprising smaller sub-sets.
As the B. barbus population of the River Teme (and the River Severn basin generally) is an important angling resource (Amat-Trigo et al., 2017), the use of stomach contents analysis via destructive sampling of the juvenile and adult fish was not possible. Consequently, trophic analyses were based on stable isotope analysis (SIA), where the ecological application of carbon (as δ13C) and nitrogen (as δ15N) stable isotopes is based on the predictable relationship between the isotope composition of a consumer and its prey. It thus provides a temporally integrative and powerful tool to analyse trophic interactions between native and non-native fishes (Cucherousset et al., 2012). For comparisons of SI data within and between the fishes, two metrics were used: the significance of differences in δ15N-δ13C centroids, and core isotopic niche sizes (as a proxy of the trophic niche) and overlaps calculated using standard ellipse areas (Jackson et al., 2011, 2012).
Methods
Sampling details and stable isotope analysis
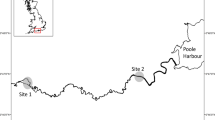
The study was conducted on the middle reaches of the River Teme, from the town of Tenbury Wells (52°19′N, − 2°24′W) to the village of Bransford (52°10′N, − 2°16′W) (Fig. 1). Across the Teme catchment, altitude varies between 24.3 and 544.5 mAOD, and land-use is primarily grassland (59%), with some horticulture (24%) (CEH, 2018). In the study reach, the river generally comprised sequences of pool and riffles, where maximum depths rarely exceeded 2 m and widths rarely exceeded 15 m. A flow gauging station towards the downstream end of the reach near Bransford had a long-term Q95 of 2.0 m3 s−1, Q50 of 10.2 m3 s−1 and Q10 of 42.4 m3 s−1 (CEH, 2018). In the study reach, the cyprinid fish community had relatively limited diversity, with only invasive B. barbus, and S. cephalus, dace Leuciscus leuciscus (Linnaeus, 1758) and minnow Phoxinus phoxinus (Linnaeus, 1758) present. Grayling Thymallus thymallus (Linnaeus, 1758) were also present in samples at the upper end of the reach and so were also included in analyses. Other species that were occasionally present in samples but not included in analyses were bullhead Cottus gobio Linnaeus, 1758 and stone loach Barbatula barbatula (Linnaeus, 1758). Brown trout Salmo trutta Linnaeus, 1758 and juvenile Atlantic salmon Salmo salar Linnaeus, 1758 are also present in the river but are more prevalent upstream of the town of Ludlow, outside of the study reach (Fig. 1). Compared with the area of river located close to the confluence with the River Severn and that was used by Gutmann Roberts et al. (2017), angling pressure was relatively light in the study reach, and thus inputs of angling baits containing high proportions of pelletized fishmeal were considered as relatively low.
Inset: location of the River Teme in Great Britain. Main map: The River Teme catchment showing its confluence with the River Severn. Arrows mark the direction of river flow. The study area was the stretch of the river between the two dashed lines. Macroinvertebrates were collected at locations marked with asterisks
The 0+ fish were sampled from a single area of nursery habitat located close to Bransford (Fig. 1). They were sampled using a micromesh seine net (25 × 2 m) on 12 September 2016. The fish were euthanised via anaesthetic overdose (MS-222) and transported back to the laboratory on ice. In the laboratory, they were identified to species, measured (standard length, nearest mm) and a sample of dorsal muscle tissue removed and dried to constant weight at 50°C. The juvenile and adult fish samples were collected using angling and electric fishing between July and September 2015 and 2016, with SIA based on scales (Busst & Britton, 2016, 2017). Correspondingly, for each captured fish, identification was to species level, followed by measuring (fork length, nearest mm) and the collection of between three and five scales from the area between the base of the dorsal fin and above the lateral line. As scales grow as fish length increases, only the outer portion of scales reflects their most recent growth (Hutchinson & Trueman, 2006; Bašić et al., 2015). Consequently, only the very outer portion of the sampled scales was used in SIA. One scale was prepared per fish, with this involving their thorough washing with distilled water, removal of the scale outer edge using dissection scissors and then drying to constant weight as per the 0+ fish samples. The other scale samples were used to age the fish (Amat-Trigo et al., 2017). Scale decalcification was not performed prior to SIA, since the removal of inorganic carbonates has no significant effect on scale δ13C and δ15N values (Sinnatamby et al., 2007, 2010; Woodcock & Walther, 2014).
Concomitantly, qualitative samples for SIA of macroinvertebrates were collected from two areas of the river, ‘Area 1′ and ‘Area 2′. Samples in Area 1 were collected from Tenbury Wells (52°19′N, − 2°24′W) and Lindridge (52°32′N, − 2°51′W), and from Area 2 at Bransford (52°10′N, − 2°16′W) in June and September 2015 and 2016. Samples were collected using kick sampling. Macro-invertebrate samples collected in 2015 contained very high proportions of the amphipod Gammarus pulex (Linnaeus, 1758) in both sampling areas (> 50%). Gammarus spp. are common prey items for riverine fishes generally (e.g. MacNeil et al., 1999), as well as the fishes analysed here more specifically (e.g. Mann, 1974; Bašić et al., 2015). Thus, samples were taken to describe the stable isotope data of fish putative prey in 2015. This sampling was repeated in 2016, with G. pulex samples taken for SIA to enable consistent temporal and spatial testing of differences in fish putative prey resources. However, to increase the diversity of these baseline samples, samples of Chironomid larvae (n = 6 per Area) and Trichoptera spp. larvae (n = 3 per Area) were also taken in 2016. All samples were taken back to the laboratory where they were washed in distilled water and dried to constant weight as per the fish samples; note that in each case, one sample comprised between three and six individuals.
The dried muscle, scale and invertebrate samples were then submitted to the Cornell Isotope Laboratory in New York, USA, for SIA. This involved the samples being ground to powder, weighed in tin capsules (nearest 1,000 µg) and analysed on a Thermo Delta V isotope ratio mass spectrometer (Thermo Scientific, USA) interfaced to a NC2500 elemental analyser (CE Elantach Inc. USA). Standards were verified against international reference materials and calibrated against the primary reference scales for δ13C and δ15N. The accuracy and precision were checked every ten samples using a standard animal sample (mink). The outputs were values of δ13C and δ15N (‰) for each sample. As C:N ratios were below 3.5, indicating low lipid content, there was no need for δ13C to be lipid corrected (Post et al., 2007; Skinner et al., 2016).
Data analysis
The 0+ fish utilised in the analysis were all between 17 and 38 mm and in their first year of life. The juvenile fish were between 86 and 231 mm and between ages 1+ and 4+ years; note that in this length range, some L. leuciscus would have been sexually mature, but with B. barbus and S. cephalus being immature. The adult fish, comprising only B. barbus and S. cephalus, were all ≥ 386 mm (Table 2). The fish ages were derived by scale ageing using a projecting microscope and accounting annual marks as per Amat-Trigo et al. (2017). For inter-specific data analyses, these length classes were considered separately. This was because the habitat use of these species tended to be quite different, with the 0+ fishes all sampled from marginal areas of the river where flows were minimal, the juvenile fishes were generally captured from relatively shallow and fast-flowing riffle habitats, and the adult fishes have relatively large home ranges in the basin, often exceeding 5 km (Hunt & Jones, 1974). By only completing inter-specific analyses within these groups of lengths, then the data were being tested between fishes of relatively similar body sizes. This meant that these analyses would be more ecologically relevant for testing the hypothesis than comparing data between species of very different length ranges (Bašic & Britton, 2015).
Prior to analysing the stable isotope analysis of the fishes, the stable isotope data of the macro-invertebrate samples were tested for spatial (Area 1 versus Area 2) and temporal (2015 vs. 2016) differences. Testing used generalized linear models (GLM) due to the relatively low sample sizes that were not normally distributed. The GLM revealed some significant differences (cf. Results). Thus, to enable the stable isotope data of the juvenile and adult fish to be combined for use across the entire study reach, their isotopic data required ‘correction’ (Jackson & Britton, 2014). Correspondingly, the δ15N data were converted to trophic position (TP; Eq. 1) and the δ13C data were corrected to CCorr (Eq. 2) (Olsson et al., 2009; Jackson & Britton, 2014). To identify the effect of this correction on the stable isotope data, differences were tested in the temporal data in the uncorrected and the corrected data of the juvenile fishes using ANOVA. The juvenile fishes were used in preference to the adult fishes for this, as the diet of the latter was also likely to have had some influence from angling baits containing marine-derived fishmeal (Bašić et al., 2015; Gutmann Roberts et al., 2017). This testing was not completed for the 0+ fishes, as their samples were taken from a single site in 2016. However, their data were also corrected to enable their results to be compared with the juvenile and adult fishes. The stable isotope correction equations were
where TPi is the trophic position of the fish, δ15Ni is the isotopic ratio of the fish, δ15Nbase is the isotopic ratio of primary consumers, 3.4 is the fractionation between trophic levels and 2 is the trophic position of the baseline organism (Post, 2002); and δ13Ccorr is the corrected carbon isotope ratio of the fish, δ13Ci is the uncorrected isotope ratio of the fish, δ13Cmeaninv is the mean invertebrate isotope ratio and CRinv is the invertebrate carbon range (δ13Cmax − δ13Cmin) (Olsson et al., 2009).
The initial analyses using the corrected SI data tested differences in TP and CCorr between species and between different life stages of the same species using ANOVA or Welch’s test, with the latter used where the data were normally distributed but violated the assumption of homogeneity of variance. For each life stage, the corrected SI data were then used to test the significance of differences in their δ15N-δ13C centroids, and differences in the positions and overlaps of their core trophic niches. For testing differences in the δ15N-δ13C centroids per life stage and species, the SIA data were normalised by square root transformation and a resemblance matrix computed using Euclidean distances (Dethier et al., 2013). A PERMANOVA model was then fitted to this distance matrix using the adonis function in the vegan package in R. This calculated the significance of the differences in δ15N-δ13C centroids per group (Oksanen et al., 2007; R Core Team, 2017). As the adonis function is similar to traditional ANOVA, it provided a pseudo F-statistic and P value based on 999 permutations of the data (Dixon, 2003). Using the same method, it was then determined whether different life stages within B. barbus and S. cephalus had significant differences in their δ15N-δ13C centroids. With more than two life stages of fish being used per test, pairwise comparisons tested the significance of differences between the groups, with Bonferroni adjustment for multiple comparisons.
To compare ‘core’ trophic niche size and overlaps within and between species, the isotopic niche was used, where the isotopic niche is an approximation of the trophic niche. It is acknowledged that the isotopic niche varies slightly from the trophic niche due to it being influenced by factors other than diet (Jackson et al., 2011), such as growth and metabolic rate of individuals (Busst & Britton, 2017). It was calculated using the metric ‘standard ellipse area’ (SEA), a bivariate measure of the distribution of individuals in isotopic space (Jackson et al., 2012). To examine the size and overlap of the ‘core’ isotopic niches of each size group by species, ellipses were plotted that enclosed 40% of the predicted data and thus the typical resource use of that life stage of fish. The ellipses were calculated within the R package SIBER v2.1.3 (Jackson et al., 2011, 2012) and, due to some relatively small sample sizes, a corrected Bayesian estimate of Standard Ellipse Area (SEAc) was calculated. This was followed by a calculation utilising a Markov chain Monte Carlo simulation with 104 iterations for each analysed group that provided 95% confidence limits (SEAb) of the isotopic niche size (Jackson et al., 2011; R Core Team, 2017). Using SEAc, the extent of niche overlap (%) between species and life stages was then also estimated. This was determined using the maximum likelihood fitted standard ellipses, with the extent of the overlap between two groups thus represented by the overlap of their core niches. This was calculated using Bayesian modelling in the SIBER package, with the denominator being the sum of non-overlapping area of the two ellipses (Jackson et al., 2011).
Results
Stable isotope correction for macro-invertebrate temporal and spatial differences
Comparison of spatial differences in SI data of the Chironomid larvae and Trichoptera spp. in 2016 revealed minimal differences in mean values, with overlaps in their 95% confidence limits (Chironomid: δ13C: Area 1: − 31.36 ± 0.39, Area 2: 31.76 ± 0.42‰; δ15N: Area 1: 9.88 ± 0.35, Area 2: 9.74 ± 0.12‰; Trichoptera: δ13C: Area 1: 32.36 ± 0.45, Area 2: 32.25 ± 0.38‰; δ15N: Area 1: 9.20 ± 0.31, Area 2: 8.86 ± 0.42‰). In G. pulex, however, some spatial and temporal differences in their SI data were apparent that, when tested in GLMs, revealed significant differences (δ13C: Wald χ2 = 12.05, P < 0.01; δ15N: Wald χ2 = 23.5, P < 0.01; Table 1A). Pairwise comparisons of the mean SI values from both models revealed these significant differences were both spatial and temporal for both stable isotopes (Table 1B, C). Consequently, the use of Eqs. 1 and 2 to correct the fish SI data used the G. pulex SI data only (Table 1A). Prior to data correction, there were significant differences in the juvenile fish stable isotope data between years (ANOVA: δ13C F1,45 = 5.05, P = 0.03; δ15N F1,45 = 11.56, P < 0.01; Fig. 2A). However, these significant differences were no longer apparent following isotopic correction (ANOVA: δ13C F1,45 = 0.10, P = 0.75; δ15N F1,45 = 1.11, P = 0.30; Fig. 2B). Note that in the tests, SI data from L. leuciscus were not included as they were only present in samples in 2016.
Intra- and inter-specific stable isotope relationships
The lengths of each species were very similar across the 0+ fishes. There was greater natural variation between the lengths of fishes as juveniles (T. thymallus were smaller than other fishes) and adults (S. cephalus were generally smaller than B. barbus) (Table 2). The only species with all life stages represented in analyses were B. barbus and S. cephalus. For B. barbus, Ccorr was significantly higher in adults than the 0+ fish and juveniles (P < 0.01), whilst TP was significantly lower for adults versus the 0+ fish (P < 0.01) (Table 3). For S. cephalus, the 0+ fish had significantly lower Ccorr than juveniles and adults (P < 0.01) and significantly higher TP (P < 0.01) (Table 3).
Between the species, differences in Ccorr between 0+ B. barbus and 0+ S. cephalus were not significant, but was between both of these 0+ fishes and 0+ P. phoxinus (P < 0.01; Table 3). The TP of 0+ B. barbus was significantly higher than both S. cephalus and P. phoxinus (P < 0.01), although it was not significantly different between S. cephalus and P. phoxinus (P > 0.05; Table 3). In juveniles, Ccorr of B. barbus was significantly lower than all other fishes (P < 0.01; Table 3). The TP of juvenile B. barbus was significantly higher than T. thymallus, significantly lower than L. leuciscus, but not significantly different to S. cephalus (Table 3). There were no significant differences in Ccorr and TP between adult B. barbus and S. cephalus (P > 0.05; Table 3).
Differences in δ15N-δ13C centroids
The overall test for differences in the positions of the δ15N-δ13C centroids of the 0+ fishes was significant (F2,58 = 15.72, P < 0.01); pairwise comparisons indicated the significant differences were between P. phoxinus and the other fishes (P < 0.01 in both cases), but with differences between B. barbus and S. cephalus being not significant (P > 0.05; Table 4). For the juvenile fishes, the overall model was significant (F3,76 = 18.41, P < 0.01), with significant differences between all species (P < 0.01; Table 4). This was in contrast to the adult B. barbus and S. cephalus, which were not significantly different (F1,41 = 1.77, P = 0.18).
The model testing differences in δ15N-δ13C centroids between the different life stages of B. barbus was significant (F2,64 = 28.89, P < 0.01), with pairwise comparisons indicating the significant differences were between adults and the other life stages (P < 0.01; Table 4). Whilst the overall model was also significant in S. cephalus (F2,49 = 17.31, P < 0.01), pairwise comparisons indicated the significant differences were only between the 0+ fishes and the other life stages (P < 0.01; Table 4).
Core isotopic niches (standard ellipse areas)
The 95% confidence intervals of the core isotopic niches (as standard ellipse areas) at each life stage suggested they were similar in size between the species (Table 5). In general, the core isotopic niches of the 0+ fishes had low overlap (maximum 7% between B. barbus and S. cephalus), the juvenile fishes had no niche overlap, but in adult B. barbus and S. cephalus, their niches overlapped by 55% (Fig. 3). Within B. barbus, there was no overlap in their core niches between the 0+, juvenile and adult fish (Fig. 3). In S. cephalus, there was no niche overlap between 0+ and juveniles, with this increasing to 2% between 0 + and adults, and then the juvenile niche sitting entirely within the adult niche (Fig. 4).
Corrected Carbon (Ccorr) versus trophic position (TP) for Barbus barbus (filled triangle), Squalius cephalus (filled square), Leuciscus leuciscus (delta) and Thymallus thymallus (open square), and the positions of their core isotopic niches (as SEAc), where solid line: B. barbus, small dashed line: S. cephalus, long dashed line: L. leuciscus, and dash/dot line: T. thymallus. (a) 0+ fishes; (b) juvenile fishes; and (c) adult fishes
Intra-specific comparisons of Corrected Carbon (Ccorr) versus trophic position (TP) and positions of core isotopic niches (as SEAc) for (a) Barbus barbus and (b) Squalius cephalus, and where filled diamond, solid line: 0+ fish; open circles, dashed line: juvenile fish; filled triangle, dotted line: adult fish
Discussion
Hypothesis 1 tested whether there were consistent inter-specific patterns of trophic partitioning between B. barbus and the other fishes. It was formulated due to these patterns of niche partitioning being evident between the fishes in the B. barbus indigenous range (Bašic & Britton, 2016). An alternative to this hypothesis would be the fishes having high niche overlap, as has been suggested between invasive and endemic Barbus fishes in Italian rivers, where it appears to have resulted in the competitive displacement of the endemics (Carosi et al., 2017). Hypothesis 1 was tested using two analyses, δ15N-δ13C centroids and core isotopic niches. The centroids were calculated using all SI data per life stage and species, whereas cores niches are based on a predicted 40% of the SI data to indicate typical resource use (Jackson et al., 2011, 2012). There were some consistent results from these analyses that aligned with Hypothesis 1, especially in the juvenile fishes where there were significant differences in δ15N-δ13C centroids between all species and no overlaps in their core niches. In the 0+ fishes, there was less consistency in the results of both analyses, with isotopic niches showing low inter-specific overlap, but with δ15N-δ13C centroids showing significant differences only between P. phoxinus and the other fishes. Whilst there was poor alignment of the results in the adult fishes with Hypothesis 1, both analyses provided consistent results; the δ15N-δ13C centroids of the adult B. barbus and S. cephalus were not significantly different and their core niches had relatively high overlap (55%). Across all life stages and analyses, there was no evidence to support the alternative hypothesis that invasion by B. barbus had resulted in competitive displacement of native fishes, as suggested by Carosi et al. (2017) for endemic Barbus. It is, however, acknowledged that this was not tested implicitly here, given the absence of data from the pre-invaded period or from sites with B. barbus absent.
The pattern of isotopic niche partitioning between B. barbus and other fishes was thus consistent with a number of isotopic studies completed on populations in their indigenous range (Bašić et al., 2015; Bašić and Britton, 2015, 2016). These studies all suggested that B. barbus and S. cephalus have distinct core isotopic niches, with minimal inter-specific resource sharing. This pattern was evident in rivers that had been stocked with hatchery-reared B. barbus at sizes below 250 mm and remained evident in adult fishes (Bašić and Britton, 2016). In contrast, Gutmann Roberts et al. (2017) revealed that in the lower reaches of the study river, there was high overlap in the core isotopic niches of adult B. barbus and S. cephalus, primarily the result of individual fishes specialising in the consumption of pelletized marine fishmeal utilised by anglers. This niche overlap was also evident in the adult fishes here, where inter-specific niche differences were only significant in the 0+ and juvenile fishes. In B. barbus, there were strong and significant patterns in niche partitioning between their different life stages, suggesting considerable ontogenetic shifts in their diet that resulted in their population having a relatively large core isotopic niche that was composed of at least three distinct sub-sets. This was consistent with Hypothesis 2, although the δ15N-δ13C centroids suggested differences were not significant between the 0+ and juvenile fish. In contrast, the isotopic niches of S. cephalus were more similar over their three studied life stages, with only the niche of the 0+ fish being distinct from the other life stages, with the juvenile and adult niches overlapping completely, contrary to Hypothesis 2.
Stable isotope data of 0+ fishes can be confounded by issues of their data still showing a strong parental signal. For example, in anadromous brown trout Salmo trutta, newly emerged fry retained a strong parental, marine-based isotopic signal that enabled their differentiation from fry produced from non-anadromous parents, but this difference was much reduced after four months of feeding in freshwater (Briers et al., 2013). In 0+ smallmouth bass Micropterus dolomieu, post-hatch embryos had elevated δ15N values that were associated with their parental origin, but these values subsequently decreased rapidly due to their exogenous feeding during their metamorphosis from larvae into juveniles (Vander Zanden et al., 1998). Here, the 0+ fishes utilised were all of lengths above 17 mm, were all fully formed juveniles rather than larvae, and were up to 10 weeks old. Their stable isotope data were very distinct from those of the adult fishes; in terms of uncorrected data, the 0+ fishes were depleted in δ13C by up to 8‰ compared to adult conspecifics. Consequently, the strong patterns of core isotopic niche partitioning detected in these 0+ fishes were interpreted as resulting from their dietary differences formed by their exogenous feeding within the river, rather than being a legacy of their parental isotopes.
In this study, the fish SI data were ‘corrected’ to enable data to be combined across two sampling years and relatively long stretch of river. The use of standard equations to ‘correct’ SI data in this manner is well established (e.g. Olsson et al., 2009; Jackson & Britton, 2014). However, this correction relies upon an adequate description of the SI data of the fish prey resources. Here, correction focused on use of G. pulex SI data. The rationale for this was they were present in all samples from all areas sampled (in contrast to other macro-invertebrate taxa), there were significant differences in their isotopic data between years and sites (whereas data from other macro-invertebrate taxa revealed similar values, at least spatially), and literature suggests Gammarus spp. are an important prey item for many fishes (e.g. MacNeil et al., 1999). Correction of the juvenile fish SI data using the G. pulex data was also demonstrated as removing significant temporal differences. To enable the SI data of the 0+ fishes to be comparable to the other fishes, they were also corrected using the G. pulex data, although stomach contents analyses (SCA) for the 0+ fishes had suggested that these amphipods were a minor prey item (Gutmann Roberts & Britton, 2018). However, individuals of > 20 mm standard length in all the 0+ fish species analysed had infections of the intestinal parasite Pomphorhynchus tereticollis (C. Gutmann Roberts, unpublished data). This parasite has gammarids as its intermediate host (Kaldonski et al., 2008), suggesting G. pulex might have been consumed in greater proportions in the 0+ fishes than suggested by SCA. Moreover, in the River Severn basin, Pomphorhynchus spp. has been reported as prevalent in all the fishes studied here (Brown 1984), suggesting gammarids are a common and important prey item of fish in the river. Therefore, although it is acknowledged that the macro-invertebrate baseline SI data used to correct the data here could have utilised a wider range of taxa, especially in 2015, it is strongly argued that the use of G. pulex SI data to correct the fish SI data was justified and appropriate.
Following introductions of non-native fishes, adverse ecological impacts often develop through increased inter-specific competition for food resources between invasive and sympatric native fishes (Gozlan et al., 2010; Cucherousset et al., 2012). Given the relatively similar size ranges of the invasive B. barbus with other cyprinid fishes across the different life stages (albeit with some inter-specific length differences within life stages), this suggests there was considerable potential for inter-specific competitive interactions, especially given the fishes were from relatively similar functional guilds (Bašic & Britton, 2016). However, the lack of overlap in the isotopic niches of the 0+ and juvenile fishes—the life stages when their abundances tend to be highest—suggested low dietary overlap, with only the isotopic niches of the adult fishes indicating some dietary overlap. Schulze et al. (2012) suggested that species within the same ecological guild can only coexist when they respond differently to limited resource availability with, for example, specialised species only persisting if their competitors are generalists. Evidence in literature supports this, with reduced trophic niche sizes in many co-existing fishes when compared to allopatry (Bolnick et al., 2010; Tran et al., 2015). In the study river, however, even where the isotopic niches of the fishes were partitioned, the niches were similarly sized. Although this suggests there had not been any niche constriction in the native fishes in B. barbus presence, it is acknowledged that this is speculative given that isotopic niche sizes of the native fishes were unable to be measured in B. barbus absence. Notwithstanding, the inter-specific isotopic niche partitioning evident in the study suggests that despite their similar ecological guilds and sharing similar habitats (especially the 0+ fishes), there were sufficient differences between the fishes in their functional traits and/or habitat utilisation to enable substantial differentiation in their core isotopic niches to occur (Robinson et al., 1993; Borcherding et al., 2013; Negus & Hoffman, 2013).
On one hand, the results here could suggest that the ecological impacts of invasive B. barbus are relatively minor in the river, as there was little evidence to suggest there was high diet similarity in the fishes at their most abundant life stages (0+ and juveniles). This inference is supported by other recent studies on native B. barbus that have revealed strong patterns of inter-specific core isotopic niche partitioning (e.g. Bašic & Britton, 2015, 2016). However, these studies were all limited to assessing trophic interactions via stable isotope analysis, with studies suggesting that when compared with other dietary analysis methods, such as stomach contents analyses, different results can occur, resulting from differences between items ingested (stomach contents) and assimilated (SIA) (e.g. Locke et al., 2013). Consequently, some caution is necessary if isotopic niche overlaps are to be used to infer the strength of competitive interactions. Moreover, invasive B. barbus can potentially result in other ecological concerns, such as causing habitat alterations, given recent work has demonstrated that in their native range, B. barbus act as ‘zoogeomorphic agents’ (Pledger et al., 2014, 2016). This is where their benthic foraging activities can reduce bed material stability, increase bedload transport, and impact microtopographic roughness and sediment structure (Pledger et al., 2014, 2016). This benthic foraging could then also impact upon aspects of the macro-invertebrate communities, such as decreased abundance via predation or reduced species richness via disturbance. However, these aspects were beyond the scope of this study and so further research is required to provide increased understandings of how B. barbus invasions affect macro-invertebrate communities and sediment structure. Finally, it should also be noted that the study river was low in fish species richness, with a cyprinid fish community comprising only four species (including B. barbus), with other fish taxa being very limited in diversity and abundance in the study reach, and with no other Barbus species present. Consequently, if B. barbus are introduced into a river with considerably higher native fish species richness, irrespective of the presence of any other Barbus species, there is the possibility that there will be a greater probability of higher niche overlaps within species in the fish community and thus higher potential for ecological impacts to result. It is therefore recommended that such introductions proceed only with caution and full risk assessment (Roy et al., 2018).
In summary, across three life stages of invasive B. barbus, there were some strong patterns of isotopic niche partitioning with native fishes, with this partitioning initially evident between some fishes during their first year of life that became strongly apparent at juvenile life stages. These invasive B. barbus thus integrated into this riverine food web via exploiting different food resources to the native fishes that facilitated their co-existence.
References
Amat-Trigo, F., C. Gutmann Roberts & J. R. Britton, 2017. Spatial variability in the growth of invasive European barbel Barbus barbus in the River Severn basin, revealed using anglers as citizen scientists. Knowledge and Management of Aquatic Ecosystems 418: 17–23.
Antognazza, C. M., D. Andreou, S. Zaccara & J. R. Britton, 2016. Loss of genetic integrity and biological invasions result from stocking and introductions of Barbus barbus: insights from rivers in England. Ecology and Evolution 6: 1280–1292.
Bašić, T. & J. R. Britton, 2015. Utility of fish scales from stock assessment surveys in stable isotope analysis for initial assessments of trophic relationships in riverine fish communities. Journal of Applied Ichthyology 31: 296–300.
Bašić, T. & J. R. Britton, 2016. Characterizing the trophic niches of stocked and resident cyprinid fishes: consistency in partitioning over time, space and body sizes. Ecology and Evolution 6: 5093–5104.
Bašić, T., J. R. Britton, M. C. Jackson, P. Reading & J. Grey, 2015. Angling baits and invasive crayfish as important trophic subsidies for a large cyprinid fish. Aquatic Sciences 77: 153–160.
Bašić, T., J. R. Britton, S. P. Rice & A. G. Pledger, 2017. Impacts of gravel jetting on the composition of fish spawning substrates: Implications for river restoration and fisheries management. Ecological Engineering 107: 71–81.
Bolnick, D. I., T. Ingram, W. E. Stutz, L. K. Snowberg, O. L. Lau & J. S. Paull, 2010. Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proceedings of the Royal Society—Biological Sciences 277: 1789–1797.
Borcherding, J., M. Dolina, L. Heermann, P. Knutzen, S. Kruger, S. Matern, R. van Treeck & S. Gertsen, 2013. Feeding and niche differentiation in three invasive gobies in the Lower Rhine, Germany. Limnologica 43: 49–58.
Briers, R. A., J. O. Waterman, K. Galt & R. N. Campbell, 2013. Population differentiation and temporal changes of carotenoid pigments and stable isotope ratios in the offspring of anadromous and non-anadromous trout Salmo trutta. Ecology of Freshwater Fish 22: 137–144.
Britton, J. R. & J. Pegg, 2011. Ecology of European barbel Barbus barbus: implications for river, fishery, and conservation management. Reviews in Fisheries Science 19: 321–330.
Britton, J. R., I. G. Cowx & G. Peirson, 2004. Sources of error in the ageing of stocked cyprinids. Fisheries Management and Ecology 11(6): 415–417.
Brown, A.F., 1984. The ecology of Pomphorhynchus laevis (Muller, 1776) (Acanthocephala) in the River Severn, England. Ph.D. thesis, University of Liverpool.
Busst, G. M. & J. R. Britton, 2016. High variability in stable isotope diet–tissue discrimination factors of two omnivorous freshwater fishes in controlled ex situ conditions. Journal of Experimental Biology 219: 1060–1068.
Busst, G. M. & J. R. Britton, 2017. Tissue-specific turnover rates of the nitrogen stable isotope as functions of time and growth in a cyprinid fish. Hydrobiologia. https://doi.org/10.1007/s10750-017-3276-2.
Carosi, A., L. Ghetti, G. La Porta & M. Lorenzoni, 2017. Ecological effects of the European barbel Barbus barbus (L., 1758) (Cyprinidae) invasion on native barbel populations in the Tiber River basin (Italy). The European Zoological Journal 84: 420–435.
CEH (2018) 54029—Teme at Knghtsford Bridge. http://nrfa.ceh.ac.uk/data/station/meanflow/54029. Accessed on 15 Feb, 18.
Copp, G. H., J. R. Britton, Z. Guo, V. R. Edmonds-Brown, J. Pegg, L. Vilizzi & P. I. Davison, 2017. Trophic consequences of non-native pumpkinseed Lepomis gibbosus for native pond fishes. Biological Invasions 19: 25–41.
Cucherousset, J. & J. D. Olden, 2011. Ecological impacts of nonnative freshwater fishes. Fisheries 36: 215–230.
Cucherousset, J., S. Boulêtreau, A. Martino, J. M. Roussel & F. Santoul, 2012. Using stable isotope analyses to determine the ecological effects of non-native fishes. Fisheries Management and Ecology 19: 111–119.
Dethier, M. N., E. Sosik, A. W. Galloway, D. O. Duggins & C. A. Simenstad, 2013. Addressing assumptions: variation in stable isotopes and fatty acids of marine macrophytes can confound conclusions of food web studies. Marine Ecology Progress Series 478: 1–14.
Dixon, P., 2003. VEGAN, a package of R functions for community ecology. Journal of Vegetation Science 14(6): 927–930.
Gozlan, R. E., J. R. Britton, I. Cowx & G. H. Copp, 2010. Current knowledge on non-native freshwater fish introductions. Journal of Fish Biology 76: 751–786.
Gutmann Roberts, C., T. Bašić, F. Amat Trigo & J. R. Britton, 2017. Trophic consequences for riverine cyprinid fishes of angler subsidies based on marine-derived nutrients. Freshwater Biology 62: 894–905.
Gutmann Roberts, C. & Britton, J.R. 2018. Quantifying trophic interactions and niche sizes of juvenile fishes in an invaded riverine cyprinid fish community. Ecology of Freshwater Fish (in press).
Hunt, P. C. & J. W. Jones, 1974. A population study of Barbus barbus (L.) in the River Severn. England. Journal of Fish Biology 6: 255–267.
Hutchinson, J. J. & C. N. Trueman, 2006. Stable isotope analyses of collagen in fish scales: Limitations set by scale architecture. Journal of Fish Biology 69: 1874–1880.
Jackson, M. C. & J. R. Britton, 2014. Divergence in the trophic niche of sympatric freshwater invaders. Biological Invasions 16: 1095–1103.
Jackson, A. L., R. Inger, A. C. Parnell & S. Bearhop, 2011. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. Journal of Animal Ecology 80: 595–602.
Jackson, M. C., I. Donohue, A. L. Jackson, J. R. Britton, D. M. Harper & J. Grey, 2012. Population-level metrics of trophic structure based on stable isotopes and their application to invasion ecology. PLoS ONE 7: e31757.
Kaldonski, N., M. J. Perrot-Minnot, S. Motreuil & F. Cézilly, 2008. Infection with acanthocephalans increases the vulnerability of Gammarus pulex (Crustacea, Amphipoda) to non-host invertebrate predators. Parasitology 135: 627–632.
Locke, S. A., G. Bulté, M. R. Forbes & D. J. Marcogliese, 2013. Estimating diet in individual pumpkinseed sunfish Lepomis gibbosus using stomach contents, stable isotopes and parasites. Journal of Fish Biology 82: 522–537.
Mann, R. H. K., 1974. Observations on the age, growth, reproduction and food of the dace, Leuciscus leuciscus (L.), in two rivers in southern England. Journal of Fish Biology 6: 237–253.
MacNeil, C., J. T. Dick & R. W. Elwood, 1999. The dynamics of predation on Gammarus spp. (Crustacea: Amphipoda). Biological Reviews 74: 375–395.
Meraner, A., A. Venturi, G. F. Ficetola, S. Rossi, A. Candiotto & A. Gandolfi, 2013. Massive invasion of exotic Barbus barbus and introgressive hybridization with endemic Barbus plebejus in Northern Italy: where, how and why? Molecular Ecology 22: 5295–5312.
Negus, M. T. & J. C. Hoffman, 2013. Habitat and diet differentiation by two strains of rainbow trout in Lake Superior based on archival tags, stable isotopes, and bioenergetics. Journal of Great Lakes Research 39: 578–590.
Oksanen, J., R. Kindt, P. Legendre, B. O’Hara, M. H. H. Stevens, M. J. Oksanen & M. Suggests, 2007. The vegan package. Community ecology package 10: 631–637.
Olsson, K., P. Stenroth, P. E. R. Nyström & W. Granéli, 2009. Invasions and niche width: does niche width of an introduced crayfish differ from a native crayfish? Freshwater Biology 54: 1731–1740.
Persson, L., J. Andersson, E. Wahlstrom & P. Eklov, 1996. Size-specific interactions in lake systems: predator gape limitation and prey growth rate and mortality. Ecology 77: 900–911.
Pledger, A. G., S. P. Rice & J. Millett, 2014. Reduced bed material stability and increased bedload transport caused by foraging fish: a flume study with juvenile barbel (Barbus barbus). Earth Surface Processes and Landforms 39: 1500–1513.
Pledger, A. G., S. P. Rice & J. Millett, 2016. Bed disturbance via foraging fish increases bedload transport during subsequent high flows and is controlled by fish size and species. Geomorphology 253: 83–93.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718.
Post, D. M., C. A. Layman, D. A. Arrington, G. Takimoto, J. Quattrochi & C. G. Montana, 2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152: 179–189.
R Core Team, 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/.
Robinson, B. W., D. S. Wilson, A. S. Margosian & P. T. Lotito, 1993. Ecological and morphological differentiation of pumpkinseed sunfish in lakes without bluegill sunfish. Evolutionary Ecology 7: 451–464.
Roy, H. E., W. Rabitsch, R. Scalera, A. Stewart, B. Gallardo, P. Genovesi, F. Essl, T. Adriaens, S. Bacher, O. Booy & E. Branquart, 2018. Developing a framework of minimum standards for the risk assessment of alien species. Journal of Applied Ecology 55: 526–538.
Schulze, T., H. Dörner, U. Baade & F. Hölker, 2012. Dietary niche partitioning in a piscivorous fish guild in response to stocking of an additional competitor–The role of diet specialisation. Limnologica-Ecology and Management of Inland Waters 42(1): 56–64.
Sinnatamby, R. N., J. E. Bowman, J. B. Dempson & M. Power, 2007. An assessment of de-calcification procedures for δ13C and δ15N analysis of yellow perch, walleye and Atlantic salmon scales. Journal of Fish Biology 70: 1630–1635.
Skinner, M. M., A. A. Martin & B. C. Moore, 2016. Is lipid correction necessary in the stable isotope analysis of fish tissues? Rapid Communications in Mass Spectrometry 30: 881–889.
Tran, T. N. Q., M. C. Jackson, D. Sheath, H. Verreycken & J. R. Britton, 2015. Patterns of trophic niche divergence between invasive and native fishes in wild communities are predictable from mesocosm studies. Journal of Animal Ecology 84: 1071–1080.
Trigo, F. A., C. Gutmann Roberts & R. J. Britton, 2017. Spatial variability in the growth of invasive European barbel Barbus barbus in the River Severn basin, revealed using anglers as citizen scientists. Knowledge & Management of Aquatic Ecosystems 418(17): 1–6.
Vander Zanden, M. J., M. Hulshof, M. S. Ridgway & J. B. Rasmussen, 1998. Application of stable isotope techniques to trophic studies of age-0 smallmouth bass. Transactions of the American Fisheries Society 127: 729–739.
Ventura, M. & E. Jeppesen, 2010. Evaluating the need for acid treatment prior to δ13C and δ15N analysis of freshwater fish scales: effects of varying scale mineral content, lake productivity and CO2 concentration. Hydrobiologia 644: 245–259.
Wheeler, A. & D. R. Jordan, 1990. The status of the barbel, Barbus barbus (L.) (Teleostie, Cyprinidae) in the United Kingdom. Jouranl of Fish Biology 37: 393–399.
Woodcock, S. H. & B. D. Walther, 2014. Trace elements and stable isotopes in Atlantic tarpon scales reveal movements across estuarine gradients. Fisheries Research 153: 9–17.
Zaccara, S., C. M. Antognazza, L. Buonerba, J. R. Britton & G. Crosa, 2014. Human-mediated contact zone between endemic and invasive Barbus species (Osteichthyes: Cyprinidae) in a regulated lowland river: genetic inferences and conservation implications. Italian Journal of Zoology 81: 571–583.
Acknowledgements
We thank the Environment Agency for their assistance with collection of scales from the juvenile fishes. CGR was supported by a studentship funded by the Severn Rivers Trust and Bournemouth University, with additional support from the Barbel Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Michael Power
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Roberts, C.G., Britton, J.R. Trophic interactions in a lowland river fish community invaded by European barbel Barbus barbus (Actinopterygii, Cyprinidae). Hydrobiologia 819, 259–273 (2018). https://doi.org/10.1007/s10750-018-3644-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3644-6