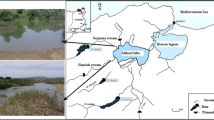
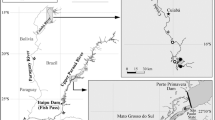
Abstract
Determining how, when, and why energy allocation occurs based on different life history traits, provides core knowledge for understanding evolution, ecology, and conservation of populations. We assumed that, in seasonal environments, Anodontites trapesialis, a common freshwater mussel in the Pantanal wetland, has to time its maturation, its larvae incubation time, and adjusts its breeding strategy seasonally. From histological analyses of gametes, larval count, and marginal increment of the shell rings, we present information about phenology and growth strategies to investigate the influence of environment and reproductive period on growth. We determined for the first time, asymptotic maximum size and longevity for this mussel. This species is a functional hermaphrodite, with maturation and spawning starting at the end of the flood period, when the water begins to recede and fishes return to the main river channel. The larvae, lasidium in this case, disperse on host fishes at this time. As we predicted, the flood pulse is the main regulatory factor to the growth patterns and reproductive period establishment. The species’ life history traits are discussed in the context of life history theory as adaptive responses to the dynamic balance imposed by the seasonality of the Pantanal.










Similar content being viewed by others
References
Anderson, M. & C. Ter Braak, 2003. Permutation tests for multi-factorial analysis of variance. Journal of Statistical Computation and Simulation 73: 85–113.
Anthony, J. L., D. H. Kesler, W. L. Downing & J. A. Downing, 2001. Length-specific growth rates in freshwater mussels (Bivalvia: Unionidae): extreme longevity or generalized growth cessation? Freshwater Biology 46: 1349–1359.
Avelar, W. & S. Mendonça, 1998. Aspects of gametogenesis of Diplodon rotundus gratus (Wagner 1827) (Bivalvia: Hyriidae) in Brazil. American Malacological Bulletin 14: 157–163.
Baird, D. G., L. R. Linton & R. W. Davies, 1986. Life-history evolution and post-reproductive mortality risk. Journal of Animal Ecology 55: 295–302.
Bauer, G., 1987. Reproductive strategy of the freshwater pearl mussel Margaritifera margaritifera. Journal of Animal Ecology 56: 691–704.
Bauer, G., 1992. Variation in the life span and size of the freshwater pearl mussel. Journal of Animal Ecology 61: 425–436.
Bauer, G., 1998. Allocation policy of female freshwater pearl mussels. Oecologia 117: 90–94.
Bayne, B. L., 2004. Phenotypic flexibility and physiological tradeoffs in the feeding and growth of marine bivalve molluscs. Integrative and Comparative Biology 44: 425–432.
Beasley, C. R., E. Tùry, W. G. Vale & C. H. Tagliaro, 2000. Reproductive cycle, management and conservation of Paxyodon syrmatophorus (Bivalvia: Hyriidae) from Tocantins River, Brazil. Journal of Molluscan Studies 66: 396–402.
Beasley, C. R., L. de Quadros Miranda, S. T. Alves, A. G. Melo, J. O. Souza & C. H. Tagliaro, 2005. Brood size and larval length of Paxyodon syrmatophorus (Bivalvia, Hyriidae) from the Tocantins River, Brazil. Amazoniana 18: 173–184.
Bertalanffy, L. Von, 1938. A quantitative theory of organic growth (inquiries on growth laws II). Human Biology 10: 181–213.
Beukema, J. J. & R. Dekker, 2005. Decline of recruitment success in cockles and other bivalves in the Wadden Sea: possible role of climate change, predation on postlarvae and fisheries. Marine Ecology Progress Series 287: 149–167.
Beukema, J. J., R. Dekker, K. Essink, & H. Michaelis, 2001. Synchronized reproductive success of the main bivalve species in the Wadden Sea: causes and consequences. Marine Ecology Progress Series 211: 143–155.
Beukema, J. J., R. Dekker & J. M. Jansen, 2009. Some like it cold: populations of the tellinid bivalve Macoma balthica (L.) suffer in various ways from a warming climate. Marine Ecology 384: 135–145.
Burnham, K. P. & D. R. Anderson, 2002. Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York: 488.
Bonetto, A. A. & I. D. Ezcurra, 1962. El desarrollo del lasidium of Anodontites trapesialis forbesianus (Lea) (Mollusca, Lamellibranchiata). Physis 23: 195–220.
Callil, C. T. & M. C. D. Mansur, 2005. Ultrastructural analysis of the shells of Anodontites trapesialis (Lamarck) and Anodontites elongatus (Swainson) (Mollusca, Bivalvia, Etherioidea) from the Mato Grosso Pantanal Region, Brazil. Revista Brasileira de Zoologia 22: 724–734.
Callil, C. T. & M. C. D. Mansur, 2007. Gametogênese e dinâmica da reprodução de Anodontites trapesialis (Lamack) (Unionoida, Mycetopodidae) no lago Baia do Poço, planície de inundação do rio Cuiabá, Mato Grosso, Brasil. Revista Brasileira de Zoologia 24: 825–840.
Callil, C. T., D. Krinski & F. A. Silva, 2012. Variations on the larval incubation of Anodontites trapesialis (Unionoida, Mycetopodidae): Synergistic effect of the environmental factors and host availability. Brazilian Journal of Biology 72: 1–8.
Coe, W. R., 1943. Sexual differentiation in mollusks. I. Pelecypods. The Quarterly Review of Biology 194: 154–164.
Colle, A. C. & C. T. Callil, 2012. Environmental influences on the composition and structure of the freshwater mussels in shallow lakes in the Cuiabá River floodplain. Brazilian Journal of Biology 72: 249–256.
da Silva Junk, W. J. C. J., C. Nunes da Cunha & K. M. Wantzen, 2011. The Pantanal: Ecology, biodiversity and sustainable management of a large neotropical seasonal wetland, 1st ed. Pensoft, Sofia: 857.
Fernandes, I. M., R. Henriques-Silva, J. Penha, J. Zuanon & P. R. Peres-Neto, 2014. Spatiotemporal dynamics in a seasonal metacommunity structure is predictable: the case of floodplain-fish communities. Ecography 37: 464–475.
Haag, W. R., 2012. North American freshwater mussels: Natural history. Ecology and Conservation, Cambridge.
Haag, W. R., 2013. The role of fecundity and reproductive effort in defining life-history strategies of North American freshwater mussels. Biological Reviews 88: 745–766.
Haag, W. R. & J. Stanton, 2003. Variation in fecundity and other reproductive traits in freshwater mussels. Freshwater Biology 48: 2118–2130.
Haag, W. R. & A. L. Rypel, 2011. Growth and longevity in freshwater mussels: Evolutionary and conservation implications. Biological Reviews 86: 225–247.
Haddon, M., 2001. Modelling and Quantitative Methods in Fisheries. Chapman and Hall, Boca Raton.
Heino, M. & V. Kaitala, 1999. Evolution of resource allocation between growth and reproduction in animals with indeterminate growth. Journal of Evolutionary Biology 12: 423–429.
Higgins, J., 2004. Introduction to Modern Nonparametric Statistics. Brooks Cole Cengage Learning, Belmont: 384.
Hunter, J. R., 1985. Preservation on Northern anchovy in formaldehyde solution. In: Lasker, R. (ed.) An egg production method for estimating spawning biomass of pelagic fish: Application to the Northernanchovy, Engraulis mordax. U.S. Dep. Comm., NOAA Tech Rep., 36: 63–66.
Jokela, J. & P. Mutikainen, 1995. Phenotypic plasticity and priority rules for energy allocation in a freshwater clam: A field experiment. Oecologia 104: 122–132.
Jones, J. W. & R. J. Neves, 2011. Influence of life-history variation on demographic responses of three freshwater mussel species (Bivalvia: Unionidae) in the Clinch River, USA. Aquatic Conservation Marine and Freshwater Ecosystems 21: 57–73.
Jones, J. W., R. J. Neves & E. M. Hallerman, 2012. Population performance criteria to evaluate reintroduction and recovery of two endangered mussel species, Epioblasma brevidens and Epioblasma capsaeformis (Bivalvia: Unionidae). Walkerana, Journal of the Freshwater Mollusk Conservation Society 35: 27–44.
Junk, W. J. & C. Nunes da Cunha, 2005. Pantanal: A large South American wetland at a crossroads. Ecological Engineering 24: 391–401.
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The flood pulse concept in river-floodplain-systems. Canadian Special Publications for Fisheries and Aquatic Sciences 106: 110–127.
Junk, W. J., M. T. F. Piedade, R. Lourival, F. Wittmann, P. Kandus, L. D. Lacerda, R. L. Bozelli, F. A. Esteves, C. Nunes da Cunha, L. Maltchik, J. Schöngart, Y. Schaeffer-Novelli & A. A. Agostinho, 2014. Brazilian wetlands: their definition, delineation, and classification for research, sustainable management, and protection. Aquatic Conservation 24: 5–22.
Kanasawa, T. & S. Sato, 2008. Environmental and physiological controls on shell microgrowth pattern of Ruditapes philippinarum (Bivalvia, Veneroidae) from Japan. Journal of Molluscan Studies 74: 89–95.
Kozłowski, J., 1993. Measuring fitness in life-history studies. Trends in Ecological and Evolution 8: 84–85.
Lindstrom, M., & D. Bates, 1990. Nonlinear mixed effects models for repeated measures data. Biometrics: 673–687.
Loverde-Oliveira, S. M. & V. L. M. Huszar, 2007. Phytoplankton ecological responses to the flood pulse in a Pantanal lake, central Brazil. Acta Limnologica Brasiliensia 19: 117–130.
Loverde-Oliveira, S., V. L. M. Huszar, N. Mazzeo & M. Scheffer, 2009. Hydrology-driven regime shifts in a shallow tropical lake. Ecosystems 12: 807–819.
McMahon, R. F. & A. E. Bogan, 2001. Mollusca Bivalvia. In Thorp, J. H. & A. P. Covich (eds.), Ecology and classification of North American freshwater invertebrates. Academic Press, San Diego.
Marçal, S. F. & S. Loverde-Oliveira, 2015. Phytoplankton in Coqueiro Lake (Pantanal de Poconé, Mato Grosso, Brazil). Biotemas 28: 9–25.
Morris, T. & L. D. Corkum, 1999. Unionid growth patterns in rivers of differing riparian vegetation. Freshwater Biology 42: 59–68.
Parada, E., S. Peredo, G. Lara & I. Valdebenito, 1989. Growth, age and life span of the freshwater mussel Diplodon chilensis (Gray, 1828). Archiv fur Hidrobiologie 115: 563–573.
Parada, E., S. Peredo & C. Gallardo, 1990. Tácticas reproductivas y dinámica poblacional de Diplodon chilensis (Gray, 1828) (Bivalvia: Hyriidae). Revista Chilena de História Natural 63: 23–35.
Peharda, M., C. A. Richardson, V. Onofri, A. Bratos & M. Crncevic, 2002. Age and growth of the bivalve Arca noae L. in the Croatian Adriatic Sea. Journal of Molluscan Studies 68: 307–310.
Penha, J., L. A. F. Mateus & J. Lobón-Cerviá, 2015. Population regulation in a neotropical seasonal wetland fish. Environmental Biology of Fishes 98: 1023–1034.
Pereira, D., M. C. D. Mansur, L. D. S. Duarte, A. S. Oliveira, D. M. Pimpão, C. T. Callil, C. Ituarte, E. Parada, S. Paredo, G. Darrigran, F. Sacarabino, C. Clavijo, L. Gladys, I. C. Miyahira, M. T. R. Rodriguez & C. Lasso, 2014. Bivalve distribution in hydrographic regions in South America: historical overview and conservation. Hydrobiologia 735: 15–44.
Roff, D. A., 1992. The Evolution of Life Histories: Theory and Analysis. Chapman and Hall, New York.
Sainmont, J., K. H. Andersen, Ø. Varpe & A. W. Visser, 2014. Capital versus income breeding in a seasonal environment. The American Naturalist 184: 466–476.
Stearns, S. C., 1977. The evolution of life history traits: A critique of the theory and a review of the data. Annual Reviews in Ecology and Systematics 8: 141–171.
Stearns, S. C., 1989. Trade-offs in life-history evolution. Functional Ecology 3: 259–268.
Stearns, S. C., 1992. The evolution of life histories. Oxford: Oxford University Press.
Stephens, P. A., I. L. Boyd, J. M. McNamara & A. I. Houston, 2009. Capital breeding and income breeding: Their meaning, measurement, and worth. Ecology 90: 2057–2067.
Varpe, Ø., C. Jørgensen, G. A. Tarling & Ø. Fiksen, 2009. The adaptive value of energy storage and capital breeding in seasonal environments. Oikos 118: 363–370.
Vaughn, C. C. & C. M. Taylor, 2000. Macroecology of a host-parasite relationship. Ecography 23: 11–20.
Vazzoler, A. E. A. M., 1996. Biologia da Reprodução de Peixes Teleósteos: Teoria e Prática. Editora da Universidade Estadual do Maringá.
Acknowledgements
We are grateful to CNPq Process No. 246223/2012-0 for the support provided during the post-doctoral and sabbatical period of C. Callil. Our thanks are also directed to the Institute of Biosciences and to the Graduate Program in Water Resources of UFMT for the financial support and logistics. We also are indebted to Manuel Lopes Lima, who invited and encouraged us to present these results at the II International Meeting of Biology and Freshwater Conservation of Bivalves, Buffalo, New York, U.S.A. Our many thanks to Professor Felipe Franco Curcio, from PPG of Zoology - UFMT, who reviewed the manuscript and made suggestions to improve it. The views expressed in this article are of the authors and do not necessarily represent those of the United States Fish and Wildlife.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Manuel P. M. Lopes-Lima, Ronaldo G. Sousa, Lyuba E. Burlakova, Alexander Y. Karatayev & Knut Mehler / Ecology and Conservation of Freshwater Bivalves
Rights and permissions
About this article
Cite this article
Callil, C.T., Leite, M.C.S., Mateus, L.A.F. et al. Influence of the flood pulse on reproduction and growth of Anodontites trapesialis (Lamarck, 1819) (Bivalvia: Mycetopodidae) in the Pantanal wetland, Brazil. Hydrobiologia 810, 433–448 (2018). https://doi.org/10.1007/s10750-017-3097-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3097-3