Abstract
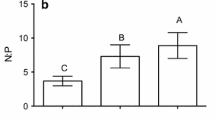
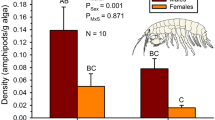
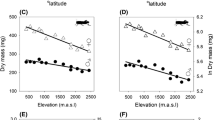
Understanding the evolution of sexually dimorphic traits requires knowledge of the genetic and environmental sources of variation. However, we know surprisingly little about how the sexes differ in their responses to environmental nutrient supply. Here, we investigated how phosphorus (P) availability, a key metric of eutrophication, affects body composition in each sex of two Hyalella amphipod species. We also examined whether differences in food preference and acquisition are responsible for observed variation in body P. We discovered environmentally-driven changes in body P that were dependent on both species and sex. In both species, males contained less P when raised in low-P laboratory conditions compared to high-P field environments, while females exhibited no significant differences. Importantly, this difference was greater in the species that is known to have larger sexual traits and higher growth rates. Variation in P content was not due to differences in acquisition of P because both sexes preferred high-P food and consumed it at a similar rate. Our study illuminates potentially important sex- and species-specific evolutionary consequences of rapid alterations to P availability due to cultural eutrophication.


Similar content being viewed by others
References
Andersson, M. B., 1994. Sexual Selection. Princeton University Press, Princeton, NJ.
APHA, 1992. Standard Methods for the Examination of Water and Wastewater. APHA, Washington, DC.
Beck, C. A., S. J. Iverson, W. D. Bowen & W. Blanchard, 2007. Sex differences in grey seal diet reflect seasonal variation in foraging behaviour and reproductive expenditure: evidence from quantitative fatty acid signature analysis. The Journal of Animal Ecology 76: 490–502.
Bertram, S. M., J. D. Schade & J. J. Elser, 2006. Signalling and phosphorus: correlations between mate signalling effort and body elemental composition in crickets. Animal Behaviour 72: 899–907.
Bertram, S. M., E. M. Whattam, L. Visanuvimol, R. Bennett & C. Lauzon, 2009. Phosphorus availability influences cricket mate attraction displays. Animal Behaviour 77: 525–530.
Boersma, M. & J. J. Elser, 2006. Too much of a good thing: on stoichiometrically balanced diets and maximal growth. Ecology 87: 1325–1330.
Bonduriansky, R., 2007. Sexual selection and allometry: a critical reappraisal of the evidence and ideas. Evolution 61: 838–849.
Bousfield, E. L., 1958. Freshwater amphipod crustaceans of glaciated North America. Canadian Field Naturalist 72: 55–113.
Brown, T., 2000. Popular Patents: America’s First Inventions from the Airplane to the Zipper. Scarecrow Press, Lanham, MD: 86 pp.
Butkas, K. J. & M. L. Ostrofsky, 2006. The status of unionid and dreissenid mussels in northwestern Pennsylvania inland lakes. The Nautilus 120: 106–111.
Clarke, J., B. Manly, K. Kerry, H. Gardner & E. Franchi, 1998. Sex differences in Adelie penguin foraging strategies. Polar Biology 20: 248–258.
Cothran, R. D. & P. D. Jeyasingh, 2010. Condition dependence of a sexually selected trait in a crustacean species complex: importance of the ecological context. Evolution 64: 2535–2546.
Cothran, R. D., A. R. Stiff, P. D. Jeyasingh & R. A. Relyea, 2012. Eutrophication and predation risk interact to affect sexual trait expression and mating success. Evolution 66: 708–719.
Elser, J. J., D. R. Dobberfuhl & N. A. MacKay, 1996. Organism size, life history, and N:P stoichiometry. BioScience 46: 674–684.
Elser, J. J., W. F. Fagan, R. F. Denno, D. R. Dobberfuhl, A. Folarin, A. Huberty, S. Interlandi, S. S. Kilham, E. McCauley, K. L. Schulz, E. H. Siemann & R. W. Sterner, 2000. Nutritional constraints in terrestrial and freshwater food webs. Nature 408: 578–580.
Elser, J. J., K. Acharya, M. Kyle, J. Cotner, W. Makino, T. Markow, T. Watts, S. Hobbie, W. Fagan, J. Schade, J. Hood & R. W. Sterner, 2003. Growth rate-stoichiometry couplings in diverse biota. Ecology Letters 6: 936–943.
Emlen, D. J. & H. F. Nijhout, 2000. The development and evolution of exaggerated morphologies in insects. Annual Review of Entomology 45: 661–708.
Fagan, W. F., E. Siemann, C. Mitter, R. F. Denno, A. F. Huberty, H. A. Woods & J. J. Elser, 2002. Nitrogen in insects: implications for trophic complexity and species diversification. The American Naturalist 160: 784–802.
Frost, P. C., J. P. Benstead, W. F. Cross, H. Hillebrand, J. H. Larson, M. A. Xenopoulos & T. Yoshida, 2006. Threshold elemental ratios of carbon and phosphorus in aquatic consumers. Ecology Letters 9: 774–779.
Gillooly, J. F., A. P. Allen, J. H. Brown, J. J. Elser, C. Martinez del Rio, V. M. Savage, G. B. West, W. H. Woodruff & H. A. Woods, 2005. The metabolic basis of whole-organism RNA and phosphorus content. Proceedings of the National Academy of Sciences of the United States of America 102: 11923–11927.
Hargrave, B. T., 1970. The utilization of benthic microflora by Hyalella azteca (Amphipoda). The Journal of Animal Ecology 39: 427–437.
Hessen, D. O., P. J. Faerovig & T. Andersen, 2002. Light, nutrients and P:C ratios in algae: grazer performance related to food quality and quantity. Ecology 83: 1886–1898.
Jaenike, J. & T. Markow, 2003. Comparative elemental stoichiometry of ecologically diverse Drosophila. Functional Ecology 17: 115–120.
Jeyasingh, P. D. & L. J. Weider, 2005. Phosphorus availability mediates plasticity in life-history traits and predator-prey interactions in Daphnia. Ecology Letters 8: 1021–1028.
Jeyasingh, P. D. & L. J. Weider, 2007. Fundamental links between genes and elements: evolutionary implications of ecological stoichiometry. Molecular Ecology 16: 4649–4661.
Jeyasingh, P. D., L. J. Weider & R. W. Sterner, 2009. Genetically-based trade-offs in response to stoichiometric food quality influence competition in a keystone aquatic herbivore. Ecology Letters 12: 1229–1237.
Jeyasingh, P. D., A. Ragavendran, S. Paland, J. A. Lopez, R. W. Sterner & J. K. Colbourne, 2011. How do consumers deal with stoichiometric constraints? Lessons from functional genomics using Daphnia pulex. Molecular Ecology 20: 2341–2352.
Kilham, S., D. Kreeger, S. Lynn, & C. Goulden, 1998. COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377: 147–159.
Lincoln, G. A., 1992. Biology of antlers. Journal of Zoology 226: 517–528.
Maklakov, A. A., S. J. Simpson, F. Zajitschek, M. D. Hall, J. Dessmann, F. Clissold, D. Raubenheimer, R. Bonduriansky & R. C. Brooks, 2008. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Current Biology 18: 1062–1066.
Markow, T. A., D. R. Dobberfuhl, C. M. Breitmeyer, J. J. Elser & E. Pfeiler, 1999. Elemental stoichiometry of Drosophila and their hosts. Functional Ecology 13: 78–84.
Markow, T. A., A. Coppola & T. D. Watts, 2001. How Drosophila males make eggs: it is elemental. Proceedings of the Royal Society of London Series B: Biological Sciences 268: 1527–1532.
Morehouse, N. I., T. Nakazawa, C. M. Booher, P. D. Jeyasingh & M. D. Hall, 2010. Sex in a material world: why the study of sexual reproduction and sex-specific traits should become more nutritionally-explicit. Oikos 119: 766–778.
Oklahoma Water Resources Board, 2005. Justification for adding nutrient limited watershed designations to water bodies in Appendix A. Oklahoma Water Resources Board, Oklahoma.
Raymond, J. & J. Himmelman, 2004. Sex differences in biochemical composition, energy content and allocation to reproductive effort in the brooding sea star Leptasterias polaris. Marine Ecology Progress 283: 179–190.
Ruckstuhl, K., 1998. Foraging behaviour and sexual segregation in bighorn sheep. Animal Behaviour 56: 99–106.
Schatz, G. S. & E. McCauley, 2007. Foraging behavior by Daphnia in stoichiometric gradients of food quality. Oecologia 153: 1021–1030.
Schindler, D. W., R. E. Hecky, D. L. Findlay, M. P. Stainton, B. R. Parker, M. J. Paterson, K. G. Beaty, M. Lyng & S. E. M. Kasian, 2008. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proceedings of the National Academy of Sciences of the United States of America 105: 11254–11258.
Shine, R., 1989. Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Quarterly Review of Biology 64: 419–461.
Smil, V., 1999. Detonator of the population explosion. Nature 400: 415.
Smil, V., 2000. Phosphorus in the environment: natural flows and human interferences. Annual Review of Energy and the Environment 25: 53–88.
Sterner, R. W. & J. J. Elser, 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton.
Sterner, R. W., T. Andersen, J. J. Elser, D. O. Hessen, J. Hood, E. McCauley & J. Urabe, 2008. Scale-dependent carbon:nitrogen:phosphorus seston stoichiometry in marine and freshwaters. Limnology and Oceanography 53: 1169–1180.
Tarnopolsky, M. A. & W. H. Saris, 2001. Evaluation of gender differences in physiology: an introduction. Current Opinion in Clinical Nutrition and Metabolic Care 4: 489–492.
Theodorou, M. E. & W. C. Plaxton, 1993. Metabolic adaptations of plant respiration to nutritional phosphate deprivation. Plant Physiology 101: 339–344.
Theodorou, M. E., I. R. Elrifi, D. H. Turpin & W. C. Plaxton, 1991. Effects of phosphorus limitation on respiratory metabolism in the green alga Selenastrum minutum. Plant Physiology 95: 1089–1095.
Tillberg, J. & J. R. Rowley, 1989. Physiological and structural effects of phosphorus starvation on the unicellular green alga Scenedesmus. Physiologia Plantarum 75: 315–324.
Visanuvimol, L. & S. M. Bertram, 2010. Dietary phosphorus availability influences female cricket lifetime reproductive effort. Ecological Entomology 35: 386–395.
Wellborn, G. A., 1994a. Size-biased predation and prey life histories: a comparative study of freshwater amphipod populations. Ecology 75: 2104–2117.
Wellborn, G. A., 1994b. The mechanistic basis of body size differences between two Hyalella (Amphipoda) species. Journal of Freshwater Ecology 9: 159–168.
Wellborn, G. A., 1995. Determinants of reproductive success in freshwater amphipod species that experience different mortality regimes. Animal Behaviour 50: 353–363.
Wellborn, G. A., 2000. Selection on a sexually dimorphic trait in ecotypes within the Hyalella azteca species complex (Amphipoda: Hyalellidae). American Midland Naturalist 143: 212–225.
Wellborn, G. A., 2002. Trade-off between competitive ability and antipredator adaptation in a freshwater amphipod species complex. Ecology 83: 129–136.
Wellborn, G. A. & S. E. Bartholf, 2005. Ecological context and the importance of body and gnathopod size for pairing success in two amphipod ecomorphs. Oecologia 143: 308–316.
Wellborn, G. A. & R. E. Broughton, 2008. Diversification on an ecologically constrained adaptive landscape. Molecular Ecology 17: 2927–2936.
Wellborn, G. A. & R. D. Cothran, 2004. Phenotypic similarity and differentiation among sympatric cryptic species in a freshwater amphipod species complex. Freshwater Biology 49: 1–13.
Wellborn, G., a., D. K. Skelly & E. E. Werner, 1996. Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecology and Systematics 27: 337–363.
Wellborn, G. A., R. Cothran & S. Bartholf, 2005. Life history and allozyme diversification in regional ecomorphs of the Hyalella azteca (Crustacea: Amphipoda) species complex. Biological Journal of the Linnean Society 84: 161–175.
Westheimer, F. H., 1987. Why nature chose phosphates. Science 235: 1173–1178.
Witt, J. D., D. L. Threloff & P. D. Hebert, 2006. DNA barcoding reveals extraordinary cryptic diversity in an amphipod genus: implications for desert spring conservation. Molecular Ecology 15: 3073–3082.
Woods, H., W. Fagan, J. Elser & J. Harrison, 2004. Allometric and phylogenetic variation in insect phosphorus content. Functional Ecology 18: 103–109.
Acknowledgments
We thank A. Buzzard and A. Ridlen for their assistance in this research. We would also like to thank several anonymous reviewers for their constructive comments on earlier versions of the manuscript. NSF Grant #0924401 to PDJ supported elemental analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: B. Oertli
Rickey D. Cothran and Punidan D. Jeyasingh are joint senior authors.
Rights and permissions
About this article
Cite this article
Goos, J.M., French, B.J., Relyea, R.A. et al. Sex-specific plasticity in body phosphorus content of Hyalella amphipods. Hydrobiologia 722, 93–102 (2014). https://doi.org/10.1007/s10750-013-1682-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1682-7