Abstract
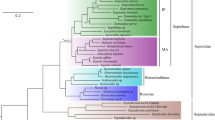
Representatives of several metazoan clades engage in symbiotic interactions with bioluminescent bacteria, but the evolution and maintenance of these interactions remain poorly understood. Uroteuthis is a genus of loliginid squid (Cephalopoda: Loliginidae) characterized by paired ventral photophores (light organs) housing bioluminescent bacteria. While previous phylogenetic studies have suggested that Uroteuthis is closely related to Loliolus, a genus of non-bioluminescent species, this relationship remains unresolved. To illuminate Uroteuthis and Loliolus phylogeny and its implications for the evolution of bioluminescence in Loliginidae, we generated sequences from two mitochondrial genes from Uroteuthis specimens sampled from several sites in the Indian and western Pacific Oceans. We combined these data with data from GenBank, analyzed the concatenated data set using maximum likelihood and Bayesian methods, and reconstructed the evolution of bacterial bioluminescence on the resulting phylogenies. Our analyses support the hypothesis that Uroteuthis is paraphyletic with respect to Loliolus. Furthermore, our reconstructions suggest that the symbiosis between loliginid squid and bioluminescent bacteria evolved once in the ancestor of Loliolini (the clade comprising Uroteuthis and Loliolus), but was subsequently lost in the ancestor of Loliolus. These findings could have profound implications for our understanding of the evolution of symbiotic bioluminescence in squid.



Similar content being viewed by others
References
Akasaki, T., M. Nikaido, K. Tsuchiya, S. Segawa, M. Hasegawa & N. Okada, 2006. Extensive mitochondrial gene arrangements in coleoid Cephalopoda and their phylogenetic implications. Molecular Phylogenetics and Evolution 38: 648–658.
Alexeyev, D. O., 1992. The systematic position of bioluminescent squids of family Loliginidae (Cephalopoda, Myopsida). Zoologicheskii Zhurnal 71: 12–23.
Anderson, F. E., 2000a. Phylogeny and historical biogeography of the loliginid squids (Mollusca: Cephalopoda) based on mitochondrial DNA sequence data. Molecular Phylogenetics and Evolution 15: 191–214.
Anderson, F. E., 2000b. Phylogenetic relationships among loliginid squids (Cephalopoda: Myopsida) based on analyses of multiple data sets. Zoological Journal of the Linnaean Society 130: 603–633.
Anderson, F. E., T. Valinassab, C. -W. Ho, K. S. Mohamed, P. K. Asokan, G. S. Rao, P. Nootmorn, C. Chotiyaputta, M. Dunning & C.- C. Lu, 2007. Phylogeography of the pharaoh cuttle Sepia pharaonis based on partial mitochondrial 16S sequence data. Reviews in Fish Biology and Fisheries 17: 345–352.
Anderson, F. E., A. Pilsits, S. Clutts, V. Laptikhovsky, E. Balguerías, G. Bello, M. Lipinski, C. Nigmatulin, J. M. F. Pereira, U. Piatkowski, J.-P. Robin, A. Salman & M. G. Tasende, 2008. Systematics of Alloteuthis (Cephalopoda: Loliginidae) based on molecular and morphometric data. Journal of Experimental Marine Biology and Ecology 364: 99–109.
Anderson, F. E., R. Engelke, K. Jarrett, T. Valinassab, K. S. Mohamed, P. K. Asokan, P. U. Zacharia, P. Nootmorn, C. Chotiyaputta & M. Dunning, 2010. Phylogeny of the Sepia pharaonis species complex (Cephalopoda: Sepiida) based on analyses of mitochondrial and nuclear DNA sequence data. Journal of Molluscan Studies 77: 65–75.
Aoki, M., H. Imai, T. Naruse & Y. Ikeda, 2008. Low genetic diversity of oval squid, Sepioteuthis cf. lessoniana (Cephalopoda: Loliginidae), in Japanese waters inferred from a mitochondrial DNA non-coding region 1. Pacific Science 62: 403–411.
Boletzky, S.v., 1970. Biological results of the University of Miami Deep-Sea Expeditions. 54. On the presence of light organs in Semirossia Steenstrup, 1887 (Mollusca: Cephalopoda). Bulletin of Marine Science 20: 374–388.
Boletzky, S.v., 1971. Neorossia n.g. pro Rossia (Allorossia) caroli Joubin, 1902, with remarks on the generic status of Semirossia Steenstrup, 1887 (Mollusca: Cephalopoda). Bulletin of Marine Science 21: 964–969.
Brakoniecki, T. F., 1986. A generic revision of the family Loliginidae (Cephalopoda: Myopsida) based primarily on the comparative morphology of the hectocotylus. Ph.D. Dissertation, The University of Miami, Coral Gables, FL.
Brakoniecki, T. F., 1996. A revision of the genus Pickfordiateuthis Voss 1953 (Cephalopoda: Myopsida). Bulletin of Marine Science 58: 9–28.
Brown, J. M., S. M. Hedtke, A. R. Lemmon & E. M. Lemmon, 2010. When trees grow too long: investigating the causes of highly inaccurate Bayesian branch-length estimates. Systematic Biology 59: 145–161.
Chun, C. 1915. Die Cephalopoden. II. Teil: Myopsida, Octopoda. Wissenschaftliche Ergebnisse der Deutschen Tiefsee-Expedition, auf dem Dampfer “Valdivia”, 1898–1899, Bd. 18. [Jena] G. Fischer.
Dai, L., X. Zheng, L. Kong & Q. Li, 2012. DNA barcoding analysis of Coleoidea (Mollusca: Cephalopoda) from Chinese waters. Molecular Ecology Resources 12: 437–447.
Darriba, D., G. L. Taboada, R. Doallo & D. Posada, 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772.
Drummond, A. J., M. A. Suchard, D. Xie & A. Rambaut, 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution 29: 3–6.
Edgar, R. C., 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5: 113.
Fitch, W. M., 1970. Distinguishing homologous from analogous proteins. Systematic Zoology 19: 99–113.
Fletcher, W. & Z. Yang, 2009. INDELible: a flexible simulator of biological sequence evolution. Molecular Biology and Evolution 26: 1879–1888.
Folmer, O., M. Black, W. Heoh, R. Lutz & R. Vrijenhoek, 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299.
Geller, J. B., E. Grosholz & G. Ruiz, 1997. Cryptic invasions of the crab Carcinus detected by molecular phylogeography. Molecular Ecology 6: 256–262.
Goldman, N., J. P. Anderson & A. G. Rodrigo, 2000. Likelihood-based tests of topologies in phylogenetics. Systematic Biology 49: 652–670.
Grabherr, M. G., B. J. Haas, M. Yassour, J. Z. Levin, D. A. Thompson, I. Amit, X. Adiconis, L. Fan, R. Raychowdhury & Q. Zeng, 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology 29: 644–652.
Guerrero-Ferreira, R. C. & M. K. Nishiguchi, 2007. Biodiversity among luminescent symbionts from squid of the genera Uroteuthis, Loliolus and Euprymna (Mollusca: Cephalopoda). Cladistics: The International Journal of the Willi Hennig Society 23: 497–506.
Guerrero-Ferreira, R. & M. Nishiguchi, 2009. Ultrastructure of light organs of loliginid squids with insights into the morphology of bacteriogenic light organs. Microscopy and Microanalysis 15: 988.
Guerrero-Ferreira, R. C. & M. K. Nishiguchi, 2010. Differential gene expression in bacterial symbionts from loliginid squids demonstrates variation between mutualistic and environmental niches. Environmental Microbiology Reports 2: 514–523.
Guerrero-Ferreira, R., C. Gorman, A. A. Chavez, S. Willie & M. K. Nishiguchi, 2012. Characterization of the bacterial diversity in Indo-West Pacific loliginid and sepiolid squid Light Organs. Microbial Ecology 65(1): 214–226. doi:10.1007/s00248-012-0099-6.
Haddock, S. H. D., M. A. Moline & J. F. Case, 2009. Bioluminescence in the sea. Annual Review of Marine Science 2: 443–493.
Heled, J. & A. J. Drummond, 2010. Bayesian inference of species trees from multilocus data. Molecular Biology and Evolution 27: 570–580.
Hudson, R. R., 1990. Gene genealogies and the coalescent process. Oxford Survey of Evolutionary Biology 7: 1–44.
Izuka, T., S. Segawa, T. Okutani & K. Numachi, 1994. Evidence on the existence of three species in the oval squid Sepioteuthis lessoniana complex in Ishigaki Island, Okinawa, southwestern Japan, by isozyme analyses. Venus 53: 217–228.
Izuka, T., S. Segawa & T. Okutani, 1996a. Identification of three species in oval squid, Sepioteuthis lessoniana complex by chromatophore arrangements on the funnel. Venus 55: 139–142.
Izuka, T., S. Segawa & T. Okutani, 1996b. Biochemical study of the population heterogeneity and distribution of the oval squid Sepioteuthis lessoniana complex in southwestern Japan. American Malacological Bulletin 12: 129–135.
Janetkitkosol, W., H. Somchanakij, M. Eiamsa-ard & M. Supongpan. 2003. Strategic review of the fishery situation in Thailand, p. 915–956. In G. Silvestre, L. Garces, I. Stobutzki, M. Ahmed, R.A. Valmonte-Santos, C. Luna, L. Lachica-Aliño, P. Munro, V. Christensen & D. Pauly (eds), Assessment, Management and Future Directions for Coastal Fisheries in Asian Countries. WorldFish Center Conference Proceedings, Vol. 67: 1–40.
Jereb, P. & C. F. E. Roper, 2006. Cephalopods of the Indian Ocean. A review. Part I. Inshore squids (Loliginidae) collected during the international Indian Ocean expedition. Proceedings of the Biological Society of Washington 119: 91–136.
Johnsen, S., E. A. Widder & C. D. Mobley, 2004. Propagation and perception of bioluminescence: factors affecting counterillumination as a cryptic strategy. The Biological Bulletin 207: 1–16.
Jones, B. W. & M. K. Nishiguchi, 2004. Counterillumination in the hawaiian bobtail squid, Euprymna scolopes Berry (Mollusca : Cephalopoda). Marine Biology 144: 1151–1155.
Kass, R. E. & A. E. Raftery, 1995. Bayes factors. Journal of the American Statistical Association 90: 773–795.
Kubatko, L. S., B. C. Carstens & L. L. Knowles, 2009. STEM: species tree estimation using maximum likelihood for gene trees under coalescence. Bioinformatics 25: 971.
Kuzuhiko, Y. & T. Kubodera, 2006. The seasonal occurrence of cephalopods caught by trap nets in coastal waters of eastern Sagami Bay. Memoirs of the National Science Museum 40: 307–315.
Lewis, P. O., 2001. A likelihood approach to estimating phylogeny from discrete morphological character data. Systematic Biology 50: 913–925.
Lindgren, A. R., G. Giribet, & M. K. Nishiguchi, 2004. A combined approach to the phylogeny of Cephalopoda (Mollusca). Cladistics: The International Journal of the Willi Hennig Society 20: 454–486.
Liu, L. & D. K. Pearl, 2007. Species trees from gene trees: reconstructing Bayesian posterior distributions of a species phylogeny using estimated gene tree distributions. Systematic Biology 56: 504–514.
Liu, L., L. Yu, D. K. Pearl & S. V. Edwards, 2009. Estimating species phylogenies using coalescence times among sequences. Systematic Biology 58: 468–477.
Lu, C. C., C. F. E. Roper & R. W. Tait, 1985. A revision of Loliolus (Cephalopoda: Loliginidae), including L. noctiluca, a new species of squid from Australian waters. Proceedings of the Royal Society of Victoria 97: 59–85.
Lum-Kong, A. & T. S. Hastings, 1992. The accessory nidamental glands of Loligo forbesi (Cephalopoda: Loliginidae): characterization of symbiotic bacteria and preliminary experiments to investigate factors controlling sexual maturation. Journal of Zoology 228: 395–403.
Maddison, W. P., 1997. Gene trees in species trees. Systematic Biology 46: 523–536.
Maddison, W. P. & D. R. Maddison. 2006. Mesquite: a modular system for evolutionary analysis, v. 1.11. http://mesquiteproject.org.
Masayuki, Y. & Y. Natsukari, 2006. Species composition and catch of loliginids, Nipponololigo (Loliolus) japonica, N. sumatrensis and N. beka, caught by commercial small trawl nets in the central Seto Inland Sea, Japan. Bulletin of the Japanese Society of Fisheries Oceanography 70: 176–179.
McFall-Ngai, M. & M. K. Montgomery, 1990. The anatomy and morphology of the adult bacterial light organ of Euprymna scolopes Berry (Cephalopoda: Sepiolidae). Biological Bulletin 179: 332–339.
McFall-Ngai, M., S. V. Nyholm & M. G. Castillo, 2010. The role of the immune system in the initiation and persistence of the Euprymna scolopes–Vibrio fischeri symbiosis. Seminars in Immunology 22: 48–53.
Montgomery, M. & M. McFall-Ngai, 1994. Bacterial symbionts induce host organ morphogenesis during early postembryonic development of the squid Euprymna scolopes. Development 120: 1719–1729.
Moynihan, M., 1983. Notes on the behavior of Euprymna scolopes (Cephalopoda: Sepiolidae). Behaviour 85: 25–41.
Naef, A. 1923. Cephalopoda. Fauna e Flora del Golfo di Napoli. Monograph No. 35. Teil I, Band 1, Lfg. 2, Fauna e Flora del Folgo di Napoli. Monograph No. 35. English translation: A Mercado (1972). Israel Program for Scientific Translations Ltd.
Natsukari, Y., 1983. Taxonomic and morphological studies on the loliginid squids: III. Nipponololigo, a new subgenus of the genus Loligo. Venus 42: 313–318.
Natsukari, Y., 1984. Taxonomical and morphological studies on the loliginid squids: IV. Two new genera of the family Loliginidae. Venus 43: 229–239.
Nishiguchi, M. K., J. E. Lopez & S.v. Boletzky, 2004. Enlightenment of old ideas from new investigations: more questions regarding the evolution of bacteriogenic light organs in squids. Evolution and Development 6: 41–49.
Okada, Y. K., 1927. Contribution a l’etude des Cephalopodes lumineux. VI. Organes photogenes des sepiolides et presence des memes organes chez Loligo edulis Hoyle. Bulletin de l’Institut Oceanographique 499: 8–15.
Pagel, M. & A. Meade, 2006. Bayesian analysis of correlated evolution of discrete characters by reversible-jump Markov chain Monte Carlo. The American Naturalist 167: 808–825.
Pagel, M., A. Meade & D. Barker, 2004. Bayesian estimation of ancestral character states on phylogenies. Systematic Biology 53: 673–684.
Rambaut, A. & A.J. Drummond. 2009. Tracer v1.5, available from http://tree.bio.ed.ac.uk/software/tracer/.
Roeleveld, M. A. C. & C. J. Augustyn, 2005. Description of a new species of Uroteuthis (Photololigo) from the Mozambique Channel. Phuket Marine Biological Center Research Bulletin 66: 97–107.
Ronquist, F., M. Teslenko, P. van der Mark, D. L. Ayres, A. Darling, S. Höhna, B. Larget, L. Liu, M. A. Suchard & J. P. Huelsenbeck, 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542.
Ruby, E. G., 1996. Lessons from a cooperative, bacterial-animal association: the Vibrio fischeri–Euprymna scolopes light organ symbiosis. Annual Review of Microbiology 50: 591–624.
Ruby, E. G., 1999. The Euprymna scolopes–Vibrio fischeri symbiosis: a biomedical model for the study of bacterial colonization of animal tissue. Journal of Molecular Microbiology and Biotechnology 1: 13–21.
Ruby, E. G., 2008. Symbiotic conversations are revealed under genetic interrogation. Nature Reviews Microbiology 6: 752–762.
Sasaki, M., 1920. Report on cephalopods collected during 1906 by the United States Bureau of Fisheries steamer Albatross in the northwestern Pacific. Proceedings of the United States National Museum 57: 163–203.
Shimomura, O., 2006. Bioluminescence: Chemical Principles and Methods. World Scientific Publishing Company Incorporated, Singapore.
Silas, E. G., R. Sarvesan, M. M. Meiyappan, K. Prabhakaran Nair, K. Satyanarayana Rao, K. Vidyasagar, Y. Appanna Sastri, P. V. Srinivasan & B. Narayana Rao, 1985. Cephalopod fisheries at selected centres in India. Central Marine Fisheries Research Institute Bulletin 37: 116–195.
Sin, Y. W., C. Yau & K. H. Chu, 2009. Morphological and genetic differentiation of two loliginid squids, Uroteuthis (Photololigo) chinensis and Uroteuthis (Photololigo) edulis (Cephalopoda: Loliginidae), in Asia. Journal of Experimental Marine Biology and Ecology 369: 22–30.
Soto, W., C. P. Lostroh & M. K. Nishiguchi, 2008. Physiological responses to stress in the Vibrionaceae: aquatic microorganisms frequently affiliated with hosts. Cooperation and stress in biology. New York, NY: Springer.
Stamatakis, A., 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690.
Steenstrup, J., 1856. Hectocotyldannelsen hos Octopodslaegterne Argonauta og Tremoctopus, oplyst ved Iagttagelse af Dannelser hos Blaeksprutterne i Alminddelighed. Danske Vidensk. Selsk. Skrift. Ser. 5(4): 187–216.
Stocsits, R. R., H. Letsch, J. Hertel, B. Misof & P. F. Stadler, 2009. Accurate and efficient reconstruction of deep phylogenies from structured RNAs. Nucleic Acids Research 37: 6184–6193.
Strugnell, J., M. Norman, J. Jackson, A. J. Drummond & A. Cooper, 2005. Molecular phylogeny of coleoid cephalopods (Mollusca: Cephalopoda) using a multigene approach; the effect of data partitioning on resolving phylogenies in a Bayesian framework. Molecular Phylogenetics and Evolution 37: 426–441.
Sundaram, S. & J. D. Sarang, 2004. Record of Loliolus investigatoris (Goodrich, 1886), Loliginid squid, Doryteuthis singhalensis (Ortmann, 1891) and Onychoteuthis baski (Leach, 1817) occurring off Mumbai waters. Marine Fisheries Information Service (Technical and Extension Series) 181: 13–14.
Swofford, D. L. 2002. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods).
Swofford, D. L., G. J. Olsen, P. J. Waddell & D. M. Hillis. 1996. Phylogenetic inference, pp. 407–514. In Molecular Systematics. Sinauer Associates Inc., Sunderland, MA.
Tomita, K., S. Yokobori, T. Oshima, T. Ueda & K. Watanabe, 2002. The cephalopod Loligo bleekeri mitochondrial genome: multiplied noncoding regions and transposition of tRNA genes. Journal of Molecular Evolution 54: 486–500.
Triantafillos, L. & M. Adams, 2005. Genetic evidence that the northern calamary, Sepioteuthis lessoniana, is a species complex in Australian waters. ICES Journal of Marine Science 62: 1665–1670.
Vecchione, M., T. F. Brakoniecki, Y. Natsukari & R. T. Hanlon, 1998. A provisional generic classification of the family Loliginidae. Smithsonian Contributions to Zoology 586: 215–222.
Vecchione, M., E. K. Shea, S. Bussarawit, F. Anderson, D. Alexeyev, C. C. Lu, T. Okutani, M. Roeleveld, C. Chotiyaputta, C. F. E. Roper, E. Jorgensen & N. Sukramongkol, 2005. Systematics of Indo-West Pacific Loliginids. Phuket Marine Biological Center Research Bulletin 66: 23–26.
Yang, Z., 2007. PAML 4: a program package for phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution 24: 1586–1591.
Young, R. E., 1977. Ventral bioluminescent countershading in midwater cephalopods. Symposia of the Zoological Society of London 38: 161–190.
Zwickl, D. J., 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Ph.D. Dissertation, The University of Texas, Austin, TX.
Acknowledgments
We would like to thank G.N. Mahardika (Universitas Udayana), Z. A. Muchlisin (Universitas Syiah Kuala), D.B. Thuy (Nha Trang University) and H.P. Calumpong (Silliman University) for their assistance in sample collection, P.H. Barber and K. Carpenter (UCLA) for funding and facilitation of sample collection, D. Willette, M. Weber and R. Rachmawati for field assistance, and Kevin Horn (SIUC) for assistance with DNA sequencing. We also thank U.S. National Science Foundation grants 1036516 (to P. H. Barber) and 0235794 (to FEA), the SIUC REACH program, the American Malacological Society, Conchologists of America, Explorer’s Club and the American Museum of Natural History Lerner-Grey Fund for Marine Research for funding and support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Erica A. G. Vidal, Mike Vecchione & Sigurd von Boletzky / Cephalopod Life History, Ecology and Evolution
Rights and permissions
About this article
Cite this article
Anderson, F.E., Bergman, A., Cheng, S.H. et al. Lights out: the evolution of bacterial bioluminescence in Loliginidae. Hydrobiologia 725, 189–203 (2014). https://doi.org/10.1007/s10750-013-1599-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1599-1