Abstract
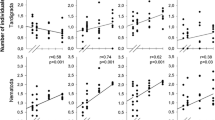
(1) A study of the metazoan community occurring in water-filled tree-holes in southern Germany has been performed to determine the relationships among the key species of arthropods found within the community and a range of structural, physical and chemical factors, using multivariate techniques. (2) Four animal species were sufficiently common to allow identification of the preferred environments for their larvae. The aedine mosquito, Aedes geniculatus, prefers shallow open tree-holes with relatively little leaf litter even though these may represent less permanent water-bodies. The scirtid beetle, Prionocyhon serricornis, occurs in larger, deeper holes with greater amounts of leaf litter and a more predictable aquatic environment, although open water is not a requisite. Larvae of the orthocladiine chironomid, Metriocnemus cavicola, favours shallow more open tree-holes with higher litter content but with sufficient open water to ensure an adequate oxygen supply. The eristaline syrphid, Myatropa florea, favours shallow, open tree-holes with low litter content. (3) There is no evidence that interspecific interactions affect the distribution or abundance of any of these species. (4) The autecological results are discussed in light of those available for phytotelm dwellers elsewhere. The food-web overall may be interpreted as so simple that it is driven by ‘bottom-up’ environmental factors with no part played by those community-level ‘top-down’ processes that may be adduced for more complex, multi-trophic level webs occurring elsewhere. No “processing chain commensalism” could be found in the arthropod community of the temperate German deciduous tree-hole dwellers.









Similar content being viewed by others
References
Barrera, R., 1996. Species concurrence and the structure of a community of aquatic insects in tree holes. Journal of Vector Ecology 21: 66–80.
Bell, T., J. A. Newman, B. W. Silverman, S. L. Turner & A. K. Lilley, 2005. The contribution of species richness and composition to bacterial services. Nature (London) 436: 1157–1160.
Bradshaw, W. E. & C. M. Holzapfel, 1986. Habitat segregation among European tree-hole mosquitoes. National Geographic Research 2: 167–178.
Bradshaw, W. E. & C. M. Holzapfel, 1988. Drought and the organization of tree-hole mosquito communities. Oecologia 74: 507–514.
Bradshaw, W. E. & C. M. Holzapfel, 1992. Resource limitation, habitat segregation, and species interaction of British tree-hole mosquitoes in nature. Oecologia 90: 227–237.
Brandt, A. v., 1934. Untersuchungen in Baumhöhlengewässern auf Fagus sylvatica. Archiv für Hydrobiologie 27: 546–563.
Carlisle, A., A. H. F. Brown & E. J. White, 1966. The organic matter and nutrient elements in the precipitation beneath a sessile oak (Quercus petraea) canopy. Journal of Ecology 54: 87–98.
Carpenter, S. R., 1982. Stemflow chemistry: effects on population dynamics of detritivorous mosquitoes in tree-hole ecosystems. Oecologia 53: 1–6.
Carpenter, S. R., 1983. Resource limitation of larval tree hole mosquitoes subsisting on beech detritus. Ecology 64: 219–22.
Eaton, J. S., G. E. Likens & F. H. Bormann, 1973. Throughfall and stemflow chemistry in a northern hardwood forest. Journal of Ecology 61: 495–508.
Fish, D. & S. L. Carpenter, 1982. Leaf litter and larval mosquito dynamics in tree-hole ecosystems. Ecology 63: 283–288.
Gallopin, G. C., 1972. Structural properties of food webs. In Patten, B. C. (ed.), Systems Analysis and Simulation in Ecology. Academic Press, New York: 241–282.
Geiser, R., 1998. Rote Liste der Käfer (Coleoptera). In Bundesamt für Naturschutz: Rote Liste gefährdeter Tiere Deutschlands, Bonn-Bad Godesberg: 75–114.
Heard, S. B., 1994. Processing chain ecology: resource condition and interspecific interactions. Journal of Animal Ecology 63: 451–464.
Jenkins, B., R. L. Kitching & S. L. Pimm, 1992. Productivity, disturbance and food web structure at a local spatial scale in experimental container habitats. Oikos 65: 249–255.
Jongman, R. H. G., C. J. F. ter Braak & O. F. R. Van Tongeren (eds), 1995. Data Analysis in Community and Landscape Ecology. Cambridge University Press.
Kitching, R. L., 1971. Water filled tree-holes and their position in the woodland ecosystem. Journal of Animal Ecology 40: 281–302.
Kitching, R. L., 1972a. The immature stages of Dasyhelea dufouri Laboulbene (Diptera: Ceratopogonidae) in water-filled tree-holes. Journal of Entomology (Series A) 47: 109–114.
Kitching, R. L., 1972b. Population studies of the immature stages of the tree-hole midge Metriocnemus martinii Thienemann (Diptera: Chironomidae). Journal of Animal Ecology 41: 53–62.
Kitching, R. L., 1983. Community structure in water-filled tree-holes in Europe and Australia—some comparisons and speculations. In Frank, J. H. & L. P. Lounibos (eds), Phytotelmata: Terrestrial Plants as Hosts of Aquatic Insect Communities. Plexus Press, Medford: 205–222.
Kitching, R. L., 2000. Food Webs and Container Habitats: The Natural History and Ecology of Phytotelmata. Cambridge University Press, Cambridge.
Kitching, R. L., 2001. Food webs in phytotelmata: “bottom-up” and “top-down” explanations for community structure. Annual Review of Entomology 46: 729–760.
Kitching, R. L. & C. Callaghan, 1982. The fauna of water-filled tree holes in box forest in south-east Queensland. Australian entomological Magazine 8: 61–70.
Kitching, R. L. & S. L. Pimm, 1985. The length of food chains: phytotelmata in Australia and elsewhere. Proceedings of the Ecological Society of Australia 14: 123–140.
Klausnitzer, B., 1984. Käfer im und am Wasser. Die Neue Brehm Bibliothek, Ziemsen, Wittenberg.
Kovac, D. & B. Streit, 1996. The arthropod community of bamboo internodes in peninsular Malasia: microzonation and trophic structure. In Edwards, D. S., W. E. Booth & S. C. Choy (eds), Tropical Rainforest Research: Current Issues. Kluwer, Dordrecht: 85–89.
Kovac, D., 1994. Die Tierwelt des Bambus: Ein Modell für komplexe tropische Lebensgemeinschaften. Natur und Museum 124: 119–136.
Kurihara, Y., 1959. Synecological analysis of the biotic community in microcosm. IV. Studies on the relations of Diptera larvae to pH in bamaboo containers. Science Reports, Tohoku University Series IV (Biology) 25: 165–171.
Mayer, K., 1938. Zur Kenntnis der Buchenhöhlenfauna. Archiv für Hydrobiologie 33: 388–400.
Mercer, D. R., 1993. Effect of tannic acid concentration on development of the western tree-hole mosquito Aedes sierrensis. Journal of Chemical Ecology 19: 1119–1127.
Palmer, M. W., 1993. Putting things in even better order: the advantages of canonical correspondence analysis. Ecology 74: 2215–2230.
Paradise, C. J., 1999. Interactive effects of resources and a processing chain interaction in tree-hole habitats. Oikos 85: 529–535.
Paradise, C. J. & W. A. Dunson, 1997a. Effects of dissolved water cations on tree-hole insect communities. Annals Entomological Society of America 90: 798–805.
Paradise, C. J. & W. A. Dunson, 1997b. Insect species interactions and resource effects in tree-holes: are helodid beetles bottom-up facilitators of midge populations? Oecologia 109: 303–312.
Paradise, C. J. & W. A. Dunson, 1998. Relationship of atmospheric deposition to the water chemistry of tree-hole habitats. Environmental Toxicology and Chemistry 17: 362–368.
Paradise, C. J. & K. L. Kuhn, 1999. Interactive effects of pH and leaf litter resources on scirtid beetles inhabiting tree-holes. Freshwater Ecology 41: 43–49.
Pavisic, V., 1941. Über die Ökologie der Baumhöhlenmückenlarven in Jugoslawien. Archiv für Hydrobiologie 33: 700–705.
Pimm, S. S. L. & R. L. Kitching, 1987. The determinants of food chain lengths. Oikos 50: 302–307.
Rohnert, U., 1950. Wassererfüllte Baumhohlen und ihre Besiedlung. Ein Beitrag zur Fauna Dendrolimnetica. Archiv für Hydrobiologie 44: 475–516.
Rotheray, G. E., 1993. Colour Guide to Hoverfly Larvae in Britain and Europe. Whiteley, Sheffield.
Schmidl, J., H. Bussler & L. Lorenz, 2003. Die Rote Liste gefährdeter Käfer Bayerns (2003) im Überblick. Bayerisches Landesamt für Umweltschutz, Beiträge zum Artenschutz 166: 87–89.
Skidmore, P., 1985. The Biology of the Muscidae of the World. Junk, Dordrecht.
Sota, T., 1996. Effect of capacity on resource input and the aquatic metazoan community structure in phytotelmata. Researches in Population Ecology 38: 65–73.
Srivastava, D. S., 2006. Habitat structure, trophic structure and ecosystem function: interactive effects in a bromeliad-insect community. Oecologia 149: 493–504.
Srivastava, D. S., J. Kolasa, J. Bengtsson, A. Gonzalez, S. P. Lawler, T. E. Miller, P. Munguia, T. Romanuk, D. C. Schneider & M. K. Trzcinski, 2004. Are natural microcosms useful model systems for ecology? Trends in Ecology and Evolution 19: 379–384.
Tate, P., 1935. The larvae of Phaonia mirabilis Ringdahl, predatory upon mosquito larvae (Diptera, Anthomyidae). Parasitology 27: 556–560.
ter Braak, C. J. F., 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67: 1167–1179.
ter Braak, C. J. F., 1988. CANOCO—a FORTRAN Programme for Canonical Community Ordination. Agricultural Mathematics Group, Wageningen.
ter Braak, C. J. F., 1990. Update Notes: CANOCO Version 3.1. Agricultural Mathematics Group, Wageningen.
ter Braak, C. J. F. & P. Smilauer, 2002. Canoco for Windows 4.5. Biometris Plant Research International, Wageningen.
Thienemann, A., 1934. Der Tierwelt der tropischen Pflanzengewässer. Archiv für Hydrobiologie Supplementum 13: 1–91.
Thienemann, A., 1954. Chironomus: Leben, Verbreitung und wirtschaftliche Bedeutung der Chironomiden. Binnengewässer 20: 1–834.
Varga, L., 1928. Ein interessanter Biotop der Biozönose von Wasserorganismen. Biologisches Zentralblatt 48: 143–161.
Walentowski, H., J. Ewald, A. Fischer, C. Kölling & W. Türk, 2004. Handbuch der natürlichen Waldgesellschaften Bayerns. Geobotanica-Verlag, Freising.
Walker, E. D. & R. W. Merritt, 1988. The significance of leaf detritus to mosquito (Diptera: Culicidae) productivity in tree holes. Environmental Entomology 17: 199–206.
Walker, E. D., D. L. Lawson, R. W. Merritt, W. T. Morgan & M. J. Klug, 1991. Nutrient dynamics, bacterial populations, and mosquito productivity in tree hole ecosystems and microcosms. Ecology 72: 1529–1546.
Yanoviak, S. P., 2001. The macrofauna of water-filled tree holes on Barro Colorado Island, Panama. Biotropica 33: 110–120.
Yanoviak, S. P. & O. M. Fincke, 2005. Sampling methods for water-filled tree holes and their artificial analogues. In Leather, S. R. (ed.), Insect Sampling in Forest Ecosystems. Blackwell: 168–185.
Yee, D. A. & S. A. Juliano, 2006. Consequences of detritus type in an aquatic microsystem: effects on water quality, micro-organisms and performance of the dominant consumer. Freshwater Biology 51: 448–459.
Acknowledgments
We thank Eva Köhnlein, Ina Wölfel, Tomi Engel and, especially, Monika Nunn for help in data collection. We are grateful, also, to Barbara Michler, Hagen Fischer and Markus Tarrasconi for comments on data analysis and to H.-W. Scheloske, University Erlangen-Nuremberg for supervising the diploma thesis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: K. Martens
Rights and permissions
About this article
Cite this article
Schmidl, J., Sulzer, P. & Kitching, R.L. The insect assemblage in water filled tree-holes in a European temperate deciduous forest: community composition reflects structural, trophic and physicochemical factors. Hydrobiologia 598, 285–303 (2008). https://doi.org/10.1007/s10750-007-9163-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-9163-5