Abstract
Refractory congestive heart failure (RCHF) is a common complication in the natural history of advanced heart failure. Peritoneal dialysis (PD) is a possible alternative in those patients, but studies are scarce, and mostly with small samples. We conducted this meta-analysis to evaluate the effects of PD in patients with RCHF. Articles published before July 2020 in the following databases: PubMed, Web of Science, and CENTRAL. Mean differences (MD) and 95% confidence intervals (CIs) were computed to generate a pooled effect size with a random effects model. We also assessed heterogeneity, risk of bias, publication bias, and quality of evidence. Twenty observational studies (n = 769) were included, with a “before and after intervention” design. PD was associated with a significant reduction in NYHA functional class (MD −1.37, 95% CI −0.78 to −1.96) and length of hospitalisation (MD −34.8, 95% CI −20.6 to −48.9 days/patient/year), a small but significant increase in left ventricular ejection fraction (MD 4.3, 95%CI 1.9 to 6.8%) and a non-significant change in glomerular filtration rate (MD −3.0, 95% CI −6.0 to 0 mL/min/1.73m2). Heterogeneity among studies was significant and overall risk of bias was rated from moderate to critical. No significant publication bias was found, and the overall quality of evidence was very low for all outcomes. PD in patients with RCHF improved functional class, length of hospitalisation, and ventricular functional, and had no impact in renal function. Further randomised clinical trials are warranted to confirm our results that showed some limitations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases are the leading cause of mortality in developed countries, including Europe [1]. Heart failure is a terminal stage in the natural history of patients with cardiovascular diseases. In the last decades, significant progress has been made in the treatment of heart failure, particularly in heart failure with reduced ejection fraction, with several disease-modifying drugs and increasingly complex devices [2, 3]. However, in some patients, the effectiveness of therapy is limited, and the only available option is palliative care to achieve some late improvement in quality of life.
In heart failure patients, congestion is a very important limiting factor for the quality of life and in very advanced stages, its control can be difficult, especially with the development of diuretic resistance, which can occur in up to 50% of hospitalised patients with acute congestive heart failure [4]. This resistance is multifactorial, and it can be related to impaired renal function, disrupted pharmacokinetics of diuretics, intravascular fluid depletion, reduced renal perfusion, activation of the renin–angiotensin–aldosterone and sympathetic systems, and compensatory distal tubular reabsorption of sodium [5].
Improvements in heart failure treatment increased survival, and refractory congestive heart failure (RCHF) is a growing health problem, being already an important cause of hospitalisation, with the associated costs [6, 7]. With diuretics resistance, extracorporeal haemodialysis or ultrafiltration is an alternative to treat congestion. However, it does not relieve the burden on hospital services, because it must be performed in a hospital setting, and clinical studies, such as the UNLOAD and CARRESS HF trial, yielded conflicting results [8, 9]. Peritoneal ultrafiltration with or without dialysis (PD) is also another alternative, with the advantage of being continuous and slow, allowing the removal of the extracellular fluid in a more physiological way, without interfering with the patient’s hemodynamic stability [4, 5]. In selected cases, it can be performed on an outpatient/home-based setting, with lower costs. Existing studies in the literature are mostly small and observational and therefore, there is lack of solid evidence on its use in heart failure. Previous systematic reviews found that hospitalisation days declined significantly, with improvements in New York Heart Association (NYHA) class and Left Ventricular Ejection Fraction (LVEF) [10, 11]. However, more recent studies were not included. For that reason, our objective is to summarise and analyse data reported in the literature, to obtain more up-to-date and consistent data on the effectiveness of PD in patients with refractory congestive heart failure.
Methods
This study was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [12]. All stages of study selection, data extraction, and quality assessment were performed independently by two reviewers. Any disagreement was resolved through discussion and consensus.
Literature search
We performed electronic database search in PubMed, Web of Science Core Collection, and Cochrane Central Register of Controlled Trials (CENTRAL) from database inception until July 2020, for articles meeting our inclusion criteria. A combination of Medical Subject Headings and text words using Boolean search strategies was used to identify studies. The following terms adapted to each database and in various combinations were used for the search: “heart failure”, “cardiac failure”, “ventricular dysfunction”, “peritoneal dialysis”, and “peritoneal ultrafiltration”. Our search did not have any language or geographical restrictions. In addition, relevant reviews obtained in the searching process as well as the references of included studies were manually analysed to search for potential additional eligible studies that were not identified in the database computer search.
Study selection
All titles and abstracts retrieved by the search were reviewed independently by two authors to identify potentially relevant articles for full-text review. Selected studies underwent full-text assessment to determine the appropriateness for inclusion.
Eligibility criteria
Inclusion and exclusion criteria were set before data extraction.
Inclusion criteria were as follows: (1) prospective or retrospective design; (2) observational cohort or randomised clinical trial design; (3) adult population (age ≥ 18 years); (4) diagnosis of refractory congestive heart failure, as defined by the 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure; (5) at least five patients treated with peritoneal dialysis; (6) pre and post-studies or comparative studies with other treatment strategies; and (7) report of at least two of the study outcomes at 6 to 12 months after initiation of PD treatment.
Exclusion criteria were as follows: reviews, editorials, letters to the editor, case reports, conference abstracts, unpublished studies, and animal experimental studies. For multiple publications from the same cohort, we chose the latest or most complete study for assessment. Studies in patients treated with PD before 1995 were also excluded.
Because this is a meta-analysis of previously published articles, ethics committee approval, and informed consent is waived.
Data extraction
Data from each study was extracted with standardised forms: first author; year of publication; country; period of enrolment; study design; mean follow-up; number of patients in each study; demographic; and study population features.
The following clinical outcomes were used to assess the efficacy of PD therapy: (1) hospitalisation duration; (2) heart function by LVEF; (3) NYHA functional classification; and (4) renal function by estimated glomerular filtration rate (GFR); we also analysed adverse clinical outcomes: peritonitis rate and all-cause mortality. The mortality rate was assessed at 1-year follow-up. Peritonitis was reported as the number of episodes per patient/year. All other outcomes were analysed as the difference before and after PD treatment.
Quality assessment
The risk of bias was independently evaluated by two authors using the Risk of Bias in Non-randomised Studies–of Interventions (ROBINS-I) tool, assessing the following domains: confounding, selection of participants, classification of intervention, deviations from the intervention, missing data, measurement of outcome, and selection of reported results [13]. These domains were qualitatively classified as at critical, serious, moderate, or low risk of bias. The overall risk of bias for each study was divided following ROBINS-I criteria. Risk of bias graphs were derived from this tool [14].
We used the Grading of Recommendations, Assessment, and Evaluation (GRADE) framework to report the overall quality and strength of the evidence per outcome [15]. The certainty in the evidence for each outcome was graded as high, moderate, low, or very low. Tables were prepared with GRADEpro™.
Statistical analysis
Statistical analyses were performed using Review Manager 5.4.1™ software. A few studies report continuous data as median and interquartile range. We used Wan and Luo formulas for imputing a missing mean and standard deviation value based on the lower quartile, median, and upper quartile summary statistics [16, 17]. They assume normally distributed outcomes but have been observed to perform well when analysing skewed outcomes [18]. A summary statistic was calculated for each study to describe the observed intervention effect. We used by default the inverse variance statistical method and the random-effects model (irrespective of the heterogeneity) to estimate pooled data. The effect measure is reported as mean difference (MD) and 95% Confidence Intervals (CI). MD represents the absolute difference between “before” and “after” intervention outcomes. Individual studies and meta-analysis estimates were derived and presented in forest plots.
Heterogeneity among studies was measured through the Cochrane’s Q test to calculate the I2 statistic that estimates the percentage of total variation between studies[19]. Based on I2, heterogeneity was rated as low (I2 < 50%), moderate (50–75%), or high (> 75%). When analysis revealed high heterogeneity, we further conducted sensitivity analysis by excluding one study at a time to reflect the effect of the specific data on the overall effect size and the stability of the results. Sensitivity analyses were also performed, by excluding studies at critical risk of bias and further excluding studies at serious and critical risk of bias.
Publication bias was assessed through visual inspection of asymmetry in funnel plots and quantitatively analysed by the Begg and Mazumdar’s rank correlation test, and the Egger’s linear regression test [20, 21]. Publication bias and publication year report were assessed with ProMeta3™ software.
A p-value < 0.05 was considered to indicate statistical significance.
Results
Included studies
The search returned 1309 records, resulting in 1178 studies after removing 131 duplicates. After title and abstract screening, 43 articles underwent full-text screening, with 20 being included for qualitative and quantitative analysis (Fig. 1) [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. There were no randomised controlled trials, and all studies have a pre- and post-intervention design. The main characteristics of the included studies are detailed in Table 1. Overall, there were 769 patients involved, mostly males, with mean age ranging from 54 to 81 years. Patients were treated from 1995 to 2017 and study’s country of origin is mainly from Europe, Middle East, and Asia. A total of 12 studies had a prospective design and all the others were retrospective. Mean follow-up ranged from 9 to 29 months.
Quality of evidence evaluation
The overall risk of bias of the included studies was rated from moderate to critical (Supplemental Figs. 1 and 2) and 60% of the studies had serious or critical risk of bias. The main reason for this classification was bias due to missing data because most studies do not report data after intervention from all subjects included at baseline—mortality rate is high, and some patients were lost to follow-up. Another important cause of bias was some deviations from the intended intervention, such as the transition to haemodialysis due to failure or complications related to PD.
No publication bias was found in most of the analysed outcomes with Begg and Mazumdar test. Funnel plots are depicted in Supplementary Fig. 3.
The GRADE confidence for all main outcomes estimates is very low (Supplemental Fig. 4).
Renal function
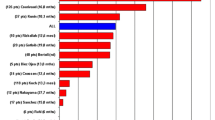
A total of 12 observational studies (n = 4 43) contributed with data for this outcome. At baseline, mean GFR ranged from 10.5 to 49.4 mL/min/1.73 m2. Pooled results showed a very small and non-significant decrease of GFR after PD initiation (MD −3.0 mL/min/1.73 m2, 95% CI −6.0 to 0, p = 0.05) (Fig. 2). Moderate statistical heterogeneity (I2 = 76%, p < 0.0001) was present for the overall pooled results. Sensitivity analysis showed that after the exclusion of the individual studies by Grosskettler, Shao, Bertoli, Nunez, and Ruhi, mean differences and 95% CI became significant, ranging from −3.1 to −3.7 mL/min/1.73 m2, all with a significant p-value. Sensitivity analysis with the removal of the critical risk of bias studies, showed a pooled effect that remained non-significant (MD −3.6, 95% CI −7.7 to 0.59, p = 0.09, I2 = 77%), as well as after removal of the serious and critical risk of bias studies (MD −0.9, 95% CI −5.9 to 4.1, p = 0.72, I2 = 18%). No publication bias was found with Egger’s test (p = 0.854) or Begg and Mazumdar’s test (p = 0.411).
Left ventricular ejection fraction
Fifteen studies (n = 562) reported LVEF before and after the intervention, with means in the range between 24 and 56% before intervention. Pooled analysis showed that after PD initiation, there was a statistically significant increase in LVEF (MD 4.33%, 95% CI 1.88 to 6.78%, p < 0.0001) (Fig. 2). There was also moderate heterogeneity (I2 = 68%, p < 0.001). Sensitivity analysis showed consistent significant differences in effect after the intervention, with increases ranging from 3.2 to 4.7%. In additional sensitivity analysis with the removal of the critical risk of bias studies, the pooled effect remained significant (MD 4.3%, 95% CI 0.81 to 7.83%, p = 0.02, I2 = 79%), but not after removal of the serious and critical risk of bias studies (MD 5.03%, 95% CI −0.12 to 10.18%, p = 0.06, I2 = 85%). No publication bias was found with Egger’s test (p = 0.764) or Begg and Mazumdar’s test (p = 0.656).
New York Heart Association (NYHA) functional class
Sixteen studies (n = 538) reported the change in NYHA class and pooled results showed a significant improvement after intervention (MD −1.37, 95% CI −0.78 to −1.96, p < 0.0001), but with very high heterogeneity (I2 = 99%, p < 0.0001) (Fig. 2). Sensitivity analysis showed persistent significant differences in effect, with reductions in NYHA functional class ranging from 1.25 to 1.44. In additional sensitivity analysis with the removal of the critical risk of bias studies, the pooled effect remained significant (MD −1.60, 95% CI −1.00 to −2.19, p < 0.00001, I2 = 99%), as well as after removal of the serious and critical risk of bias studies (MD −1.31, 95% CI −0.81 to −1.81, p < 0.0001, I2 = 90%). Significant publication bias was found with Egger’s test (p = 0.012) but not with Begg and Mazumdar’s test (p = 0.126). Publication year had a significant impact on effect size, with smaller improvements in NYHA in more recent studies (Supplemental Fig. 5).
Length of hospitalisation
A total of 10 observational studies (n = 374) reported the length of hospitalisation as days of hospitalisation/patient/year, with means ranging from 31.6 to 139.2. Pooled results showed a significant decrease after PD initiation (MD −34.8 days/patient/year, 95% CI −20.6 to 48.9, p < 0.0001) (Fig. 2). High statistical heterogeneity (I2 = 92%, p < 0.0001) was present for the overall pooled results. No individual study had a substantial impact on the pooled effect size, ranging from –30.06 to −38.08 days. In addition, sensitivity analysis with the removal of the critical risk of bias studies showed that the pooled effect remained significant (MD 49.9, 95% CI 29.1 to 70.7, p < 0.00001, I2 = 91%), as well as after the removal of the serious and critical risk of bias studies (MD 52.1, 95% CI 22.7 to 81.6, p = 0.0005, I2 = 92%). The funnel plot demonstrated slight asymmetry, suggesting a possible publication bias. However, neither Egger’s test (p = 0.348) nor Begg’s test (p = 0.655) revealed evidence of publication bias. Three studies (n = 169) reported results as days of hospitalisation/patient/month and were analysed separately, also showing a significant reduction of 3 days (Fig. 2).
Adverse clinical outcomes at 1 year
All-cause mortality at 1 year is reported in 17 studies and a mean value of 37.6% was obtained (Table 2). The other studies did not report mortality or only considered for the study patients that survived at least 12 months. Incidence of peritonitis, one of the most common complications of PD, is reported in 10 studies, and it ranged from 0 to 0.75 episodes/patient/year (Table 2).
Discussion
In this updated meta-analysis on the efficacy of PD in adult patients with RCHF, we retrieved 20 studies, representing a total of 769 patients. All were observational and non-randomised. When measured by the NYHA functional class, almost all studies showed that PD improved symptoms. There was also a positive effect on LVEF with improvements in the range between 1 and 19%. Another important benefit was a significant decline in hospitalisation days by almost 35 days/patient/year. Renal function remained stable during PD treatment, suggesting that it can avoid or delay further deterioration in renal function.
With effective control of volume overload and congestion, it is possible to reduce hospitalisations due to congestion, which we confirmed in our meta-analysis. This reduction can be considered an indirect marker of improved quality of life in these patients and a surrogate marker of better control of heart failure symptoms. We also confirmed a reduction in NYHA functional class. Moreover, as Sanchez demonstrated, total healthcare costs associated with PD were lower when compared to conservative therapy [37]. PD is also associated with a higher utility than the conservative therapy. Cost-utility for PD was, at that time, 23 305€/quality-adjusted life-year (QALY), while for conservative treatment it was 81 053€/QALY, with a difference of 46 237€ per QALY. PD is cost-effective compared with the conservative therapy and this is very important when the economic burden of heart failure is expected to increase in the next years.
We observed a slight improvement in left ventricular function. Effective decongestion by PD decreases preload that can theoretically improve ejection fraction, not only by allowing a reduction in the activation of both renin–angiotensin–aldosterone axis and sympathetic nervous system but also by another possible contributing factor related to the removal of myocardial depressant factors [26].
Renal function remained stable after PD in patients without end-stage chronic kidney disease. This may be related to improvement in renal perfusion, secondary to improved cardiac function and reduced neurohormonal activation [10, 26]. This can also be related to a reduction in renal venous congestion, with general improvement in renal hemodynamics [10, 26].
Patients with refractory congestive heart failure have a very ominous prognosis, not only in the quality of life but also in survival. Our population of patients had multiple previous hospitalisations for congestive heart failure. Previous studies showed a direct increase in all-cause mortality with the increase in the number of hospitalisations for heart failure. In a patient database of almost 15,000 patients hospitalised for heart failure between 2000 and 2004, 1-year mortality was 34% after the first hospitalisation, reaching 50% after the third hospitalisation [42]. More recent data (2007–2011), showed some improvement, being 27% at 1-year after first hospitalisation and 40% after the third hospitalisation [43]. Similar data is reported in another study with all-cause mortality at 1 year of 36.8–45.2% in patients with recurrent hospitalisations for acute decompensated heart failure, particularly in patients with heart failure with reduced ejection fraction [44]. Our meta-analysis reported a pooled mortality rate slightly lower, of 37.2%, when compared to this historical mortality rate, suggesting that this strategy possibly does not have a very significant impact on survival.
The most common complication of PD is peritonitis, and our results seem to be in line with those reported for chronic PD in end-stage kidney disease patients. In the general population of patients submitted to PD, peritonitis rates in recent publications are reported between 0.26 and 0.37 episodes/patient/year, depending on the technique used—higher for continuous ambulatory peritoneal dialysis [45,46,47]. Our results have a wide range of incidence, from 0 to 0.75 episodes/patient/year, but most are below 0.32 episodes/patient/year, particularly for studies after 2014, suggesting that this technique is currently safe (regarding infection) in patients with refractory congestive heart failure.
As in the previous meta-analysis, there are important limitations. The overall quality of most studies is poor. They were all observational; length of follow-up was also highly variable; all studies had a pre- and post-intervention design and outcomes of patients that died or were lost to follow-up for any other reason, were not reported. Implications of missing outcome data from those participants are expected to be significant, mainly because they were probably the sickest ones, and a direct comparison between pre- and post-intervention data is not advisable. Missing values were one of the main reasons for the high risk of bias given for most studies. However, analysing only the study outcomes reported in the studies where it was possible to extract specific information from the subset of patients who report both baseline and post-intervention measurements, the null effect in glomerular filtration rate is consistent, as well as the positive impact on NYHA functional class and length of hospitalisation and the effect in LVEF is either neutral or positive supporting the validity of our results [24, 26, 27, 30, 34,35,36,37, 39, 40].
There was also high heterogeneity of the pooled studies for most outcomes explained by differences regarding sample size and baseline characteristics between studies. A recent study showed that hospitalisation reductions were only significant in patients with heart failure with preserved ejection fraction and significant improvement in LVEF was only observed in patients with heart failure with reduced ejection fraction, showing the impact of heterogeneity [48].
There are other limitations. Some patients received haemodialysis due to failure of PD treatment and this is another cause for increased risk of bias. Most studies do not report appropriately pharmacological treatment or devices used in the treatment of heart failure, and for that reason, we cannot confirm if the observed change in the clinical outcome can be solely attributed to PD treatment. However, there was a consistent improvement in most outcomes which is something we do not expect in patients with such ominous prognosis.
The lack of prospective randomised controlled trials is also relevant. The peritoneal dialysis in patients with severe heart failure (PD-HF) trial, a multicentre randomised controlled trial of intermittent ultrafiltration by PD plus best standard care versus best standard care for the treatment of RCHF and moderate chronic kidney disease (stages 3–4), was initiated in 2016[49]. Over a 2-year inclusion period, only 10 patients were recruited, and the study was terminated due to the inability to recruit an adequate number of participants. The main reasons reported for ineligibility were fluctuating GFR, sub-optimal heart failure treatment, frailty, and patients being too unwell for randomisation (some patients were considered only when they were at end of life), unwillingness to engage in an invasive therapy, and suboptimal coordination between cardiology and renal services. This example shows the difficulties in engaging a randomised controlled trial, and for the time being, only the evidence presented in meta-analysis is available.
In conclusion, peritoneal dialysis/ultrafiltration in patients with refractory congestive heart failure improved functional class, length of hospitalisation, and left ventricular ejection fraction and had no impact in renal function. These favourable results can also have a very positive economic effect, but further studies on this topic are required. Moreover, randomised clinical trials are warranted to compare this intervention with pharmacological therapy or other treatment strategies regarding survival benefits or symptomatic improvement. This is essential to provide more robust evidence on the best therapeutic option in refractory congestive heart failure because there were important limitations in the studies included.
References
Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE et al (2020) European Society of Cardiology: cardiovascular disease statistics 2019. Eur Heart J 41:12–85
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS et al (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 37:2129–2200
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM et al (2017) 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 136:e137–e161
Gupta R, Testani J, Collins S. (2019) Diuretic resistance in heart failure. Curr Heart Fail Rep 16:57–66
Mullens W, Damman K, Harjola VP, Mebazaa A, Rocca HB, Martens P et al (2019) The use of diuretics in heart failure with congestion — a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 21:137–155
Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW et al (2021) Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation 143:e254–e743
Seferovic P, Vardas P, Jankowska EA, Maggioni AP, Timmis A, Milinković I et al (2021) The Heart Failure Association Atlas: heart failure epidemiology and management statistics 2019. Eur J Heart Fail [E-pub ahead of print]. https://doi.org/10.1002/ejhf.2143
Constanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR et al (2007) Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure (UNLOAD trial). J Am Coll Cardiol 49:675–683
Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA et al (2012) Ultrafiltration in decompensated heart failure with cardiorenal syndrome (CARRESS-HF trial). N Engl J Med 367:2296–2304
Lu R, Mucino-Bermejo MJ, Ribeiro LC, Tonini E, Estremadoyro C, Samoni S et al (2015) Peritoneal dialysis in patients with refractory congestive heart failure: a systematic review. Cardiorenal Med 5:145–156
Chionh CY, Clementi A, Poh CB, Finkelstein FO, Cruz DN (2020) The use of peritoneal dialysis in heart failure: a systematic review. Perit Dial Intern 40:527–539
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed) 339:b2700
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ 355:i4919
McGuinness LA, Higgins JPT (2021) Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 12:55–61
Schünemann HJOA, Higgins JPT, Vist GE, Glasziou P, Akl E, Guyatt GH (2017) on behalf of the Cochrane GRADEing methods group and the Cochrane statistical methods group. Chapter 11: completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: J.P.T.C.R. Higgins, J. Chandler, M.S. Cumpston, editors. Cochrane Handbook for Systematic Reviews of Interventions version 520 (updated June 2017), Cochrane; 2017. Available from: www.training.cochrane.org/handbook
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range, and/or interquartile range. BMC Med Res Methodol 14:135
Luo D, Wan X, Liu J, Tong T (2018) Optimally estimating the sample mean from the sample size, median, mid-range and/or mid-quartil range. Stat Methods Med Res 27:1785–1805
Weir CJ, Butcher I, Assi V, Lewis SC, Murray GD, Langhorne P, Brady MC (2018) Dealing with missing standard deviation and mean values in meta-analysis of continuum outcomes: a systematic review. BMC Med Res Methodol 18:25
Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Statist Med 21:1539–1558
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Grossekettler L, Schmack B, Meyer K, Brockmann C, Wanninger R, Kreusser MM et al (2019) Peritoneal dialysis as therapeutic option in heart failure patients. ESC Heart Failure 6:271–279
Wojtaszek E, Grzejszczak A, Niemczyk S, Malyszko J, Matuszkiewicz-Rowińska J (2019) Peritoneal ultrafiltration in the long-term treatment of chronic heart failure refractory to pharmacological therapy. Front Physiol 10:310
Shao Q, Xia Y, Zhao M, Liu J, Zhang Q, Jin B et al (2018) Effectiveness and safety of peritoneal dialysis treatment in patients with refractory congestive heart failure due to chronic cardiorenal syndrome. Biomed Res Int. https://doi.org/10.1155/2018/6529283
Pavo N, Yarragudi R, Puttinger H, Arfsten H, Strunk G, Bojic A et al (2018) Parameters associated with therapeutic response using peritoneal dialysis for therapy refractory heart failure and congestive right ventricular dysfunction. PLoS ONE 13:e0206830. https://doi.org/10.1371/journal.pone.0206830
Hedau S, Chakravarthi R, Reddy V (2018) Ultrafiltration by peritoneal route in refractory chronic congestive cardiac failure. Indian J Nephrol 28:298–302
Querido S, Branco P, Sousa H, Adragão T, Aguiar C, Pereira S et al (2016) Peritoneal dialysis as a successful treatment in patients with refractory congestive heart failure: a one-center experience. Clin Nephrol 85:260–265
Fröhlich H, Katus HA, Täger T, Lossnitzer N, Grossekettler L (2015) Peritoneal ultrafiltration in end-stage chronic heart failure. Clin Kidney J 8:219–225
Bertoli SV, Musetti C, Ciurlino D, Basile C, Galli E, Gambaro G (2014) Peritoneal ultrafiltration in refractory heart failure: a cohort study. Perit Dial Inter 34:64–70
Courivaud C, Kazory A, Crépin T, Azar R, Bresson-Vautrin C (2014) Peritoneal dialysis reduces the number of hospitalization days in heart failure patients refractory to diuretics. Perit Dial Intern 34:100–108
Rizkallah J, Sood MM, Reslerova M, Cordova F, Malik A, Sathianathan A (2013) Reduced hospitalizations in severe, refractory congestive heart failure with peritoneal dialysis: a consecutive case series. Clin Nephrol 80:334–341
Kunin M, Arad M, Dinour D, Freimark D, Holtzman EJ (2013) Peritoneal dialysis in patients with refractory congestive heart failure: potential prognostic factors. Blood Purif 35:285–294
Nunez J, Gonzalez M, Minana G, Garcia-Ramon R, Sanchis J, Bodı V (2012) Continuous ambulatory peritoneal dialysis as a therapeutic alternative in patients with advanced congestive heart failure. Eur J Heart Fail 14:540–548
Ruhi C, Kocak H, Yavuz A, Suleymanlar G, Ersoy FF (2012) Use of peritoneal ultrafiltration in the elderly refractory congestive heart failure patients. Int Urol Nephrol 44:963–969
Koch M, Haastert B, Kohnle M, Rump LC (2012) Peritoneal dialysis relieves clinical symptoms and is well tolerated in patients with refractory heart failure and chronic kidney disease. Eur J Heart Fail 14:530–539
Sotirakopoulos NG, Kalogiannidou IM, Tersi ME, Mavromatidis KS (2011) Peritoneal dialysis for patients suffering from severe heart failure. Clin Nephrol 76:124–129
Sanchez JE, Ortega T, Rodriguez C, Diaz-Molina B, Martin M (2010) Efficacy of peritoneal ultrafiltration in the treatment of refractory congestive heart failure. Nephrol Dial Transplant 25:605–610
Cnossen TT, Kooman JP, Konings CJAM, Uszko-Lencer NHMK (2010) Peritoneal dialysis in patients with primary cardiac failure complicated by renal failure. Blood Purif 30:146–152
Nakayama M, Nakano H, Nakayama M (2010) Novel therapeutic option for refractory heart failure in elderly patients with chronic kidney disease by incremental peritoneal dialysis. J Cardiology 55:49–54
Díez-Ojea B, Rodríguez-Suárez C, Vidau P, Gago E, Díaz-Molina B (2007) Papel de la diálisis peritoneal en el tratamento de la insuficiencia cardíaca. Experiencia en nuestro centro Nefrologia 7:605–611
Gotloib L, Fudin R, Yakubovich M, Vienken J (2005) Peritoneal dialysis in refractory end-stage congestive heart failure: a challenge facing a no-win situation. Nephrol Dial Transplant 20 [Suppl 7]: vii32–6
Stoguchi S, Stevenson LW, Schneeweiss S (2007) Repeated hospitalizations predict mortality in the community populations with heart failure. Am Heart J 154:260–266
Lin A, Chin JC, Scignano NM, Evans AM (2017) Repeat hospitalization predicts mortality in patients with heart failure. Mil Med 182:e1932–e1937
Chang P, Wruck LM, Shahar E, Rossi JS, Loehr LR, Russell SD et al (2018) Trends in hospitalization and survival of acute decompensated heart failure in 4 US communities (2005–2014). ARIC Study Community Surveillance Circulation 138:12–24
Perl J, Fuller DS, Bieber BA, Boudville N, Kanjanabuch T, Ito Y et al (2020) Peritoneal dialysis-related infection rates and outcomes: results from the peritonal dialysis outcomes and practice patterns study (PDOPPS). Am J Kidney Dis 76:42–53
Fiel D, Santos J, Vicente R (2020) Predictors of peritonitis in peritoneal dialysis: experience during three decades. Port J Nephrol Hypert 34:14–20
El-Reshaid W, Al-Disawy H, Nassef H, Alhelaly U (2016) Comparison of peritonitis rates and patient survival in automated and continuous ambulatory peritoneal dialysis: a 10-year single center experience. Ren Fail 38:1187–1192
Grossekettler L, Schmack B, Brockmann C, Wanninger R, Kreusser MM, Frankenstein L et al (2020) Benefits of peritoneal ultrafiltration in HFpEF and HFrEF patients. BMC Nephrol 21:179
Dukka H, Kalra PA, Wilkie M, Bhandari S, Davies SJ, Barratt J et al (2019) Peritoneal ultrafiltration for heart failure: lessons from a randomized controlled trial. Perit Dial Int 39:468–469
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
Ana Teresa Timóteo had the idea for the study, performed the literature search and data analysis, drafted, and critically reviewed the work. Tania Mano performed the literature search and critically reviewed the work.
Corresponding author
Ethics declarations
Ethical approval
The manuscript does not contain clinical studies or patient data (it is a meta-analysis of previous published studies).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Timóteo, A.T., Mano, T.B. Efficacy of peritoneal dialysis in patients with refractory congestive heart failure: a systematic review and meta-analysis. Heart Fail Rev 28, 1053–1063 (2023). https://doi.org/10.1007/s10741-023-10297-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-023-10297-3