Abstract
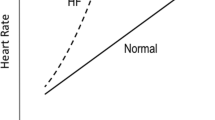
In heart failure (HF) patients, the pathophysiological mechanisms of severe exercise intolerance and impaired exercise capacity are related to both central and peripheral abnormalities. The central abnormalities in HF patients include impaired cardiac function and chronotropic incompetence (CI). Indeed, CI, the inability to adequately increase heart rate (HR) from rest to exercise often exhibited by HF patients, is related to activation of the sympathetic nervous system (SNS) yielding a rise in circulating norepinephrine (NE). CI may result from downregulation of β-adrenergic receptors, β-blocker usage, high baseline HR, or due to a combination of factors. This paper discusses the role of elevated NE in altering chronotropic responses in HF patients and consequently resulting in impaired exercise capacity. We suggest that future research should focus on the potential treatment of CI with rate-adaptive pacing, using a sensor to measure physical activity, without inducing deleterious hormonal activation of the sympathetic system.


Similar content being viewed by others
References
Myers J (2008) Principles of exercise prescription for patients with chronic heart failure. Heart Fail Rev 13:61–68
Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ (2003) American Heart Association Committee on exercise r and prevention. Exercise and heart failure: a statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation 107:1210–1225
Gupte AA, Hamilton DJ (2016) Exercise intolerance in heart failure with preserved ejection fraction. Methodist Debakey Cardiovasc J 12:105–109
Piepoli MF, Guazzi M, Boriani G, Cicoira M, Corra U, Dalla Libera L, Emdin M, Mele D, Passino C, Vescovo G, Vigorito C, Villani GQ, Agostoni P, Working Group Exercise Physiology SC and Cardiac Rehabilitation ISoC (2010) Exercise intolerance in chronic heart failure: mechanisms and therapies Part I. EurJ Prev Cardiol 17:637–642
Brubaker PH, Kitzman DW (2007) Prevalence and management of chronotropic incompetence in heart failure. Curr Cardiol Rep 9:229–235
Jorde UP, Vittorio TJ, Kasper ME, Arezzi E, Colombo PC, Goldsmith RL, Ahuja K, Tseng CH, Haas F, Hirsh DS (2008) Chronotropic incompetence, beta-blockers, and functional capacity in advanced congestive heart failure: time to pace?. Eur J Heart Fail 10:96–101
Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW (2006) Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardpulm Rehabil 26:86–89
Cohn JN, Johnson GR, Shabetai R, Loeb H, Tristani F, Rector T, Smith R, Fletcher R (1993) Ejection fraction, peak exercise oxygen consumption, cardiothoracic ratio, ventricular arrhythmias, and plasma norepinephrine as determinants of prognosis in heart failure The V-HeFT VA Cooperative Studies Group. Circulation 87:VI5-16
Corra U, Mezzani A, Bosimini E, Giannuzzi P (2004) Cardiopulmonary exercise testing and prognosis in chronic heart failure: a prognosticating algorithm for the individual patient. Chest 126:942–950
Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, Wilson JR (1991) Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 83:778–786
Lund LH, Aaronson KD, Mancini DM (2005) Validation of peak exercise oxygen consumption and the Heart Failure Survival Score for serial risk stratification in advanced heart failure. Am J Cardiol 95:734–741
Azarbal B, Hayes SW, Lewin HC, Hachamovitch R, Cohen I, Berman DS (2004) The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol 44:423–430
Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH (1999) Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA, J Am Med Assoc 281:524–529
Lauer MS, Okin PM, Larson MG, Evans JC, Levy D (1996) Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation 93:1520–1526
Samejima H, Omiya K, Uno M, Inoue K, Tamura M, Itoh K, Suzuki K, Akashi Y, Seki A, Suzuki N, Osada N, Tanabe K, Miyake F, Itoh H (2003) Relationship between impaired chronotropic response, cardiac output during exercise, and exercise tolerance in patients with chronic heart failure. Jpn Heart J 44:515–525
Schrier RW, Abraham WT (1999) Hormones and hemodynamics in heart failure. N Engl J Med 341:577–585
Kumagai H, Oshima N, Matsuura T, Iigaya K, Imai M, Onimaru H, Sakata K, Osaka M, Onami T, Takimoto C, Kamayachi T, Itoh H, Saruta T (2012) Importance of rostral ventrolateral medulla neurons in determining efferent sympathetic nerve activity and blood pressure. Hypertens Res 35:132–141
Underwood CF, Lynch EA (2017) Under what circumstances do rostral ventrolateral medulla neurons support blood pressure?. J Neurosci 37:8048–8050
Aimo A, Saccaro LF, Borrelli C, Fabiani I, Gentile F, Passino C, Emdin M, Piepoli MF, Coats AJS, Giannoni A (2021) The ergoreflex: how the skeletal muscle modulates ventilation and cardiovascular function in health and disease. Eur J Heart Fail 23:1458–1467
Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ, Piepoli MF (2001) Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation 104:2324–2330
Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J (2009) The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 54:1747–1762
Loebe M, Koster A, Sanger S, Potapov EV, Kuppe H, Noon GP, Hetzer R (2001) Inflammatory response after implantation of a left ventricular assist device: comparison between the axial flow MicroMed DeBakey VAD and the pulsatile Novacor device. ASAIO J (Am Soc Artif Internal Organs : 1992) 47:272–274
Communal C, Singh K, Pimentel DR, Colucci WS (1998) Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation 98:1329–1334
Mann DL, Kent RL, Parsons B, Cooper G 4th (1992) Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation 85:790–804
Simpson P (1983) Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J Clin Investig 72:732–738
Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T (1984) Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311:819–823
Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB (1982) Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med 307:205–211
Wever-Pinzon O, Fang JC (2019) Characterization of sympathetic innervation in heart failure with preserved ejection fraction. J Card Fail 25:314–315
Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP (2002) Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 288:2144–2150
Borrelli C, Gentile F, Sciarrone P, Mirizzi G, Vergaro G, Ghionzoli N, Bramanti F, Iudice G, Passino C, Emdin M, Giannoni A (2019) Central and obstructive apneas in heart failure with reduced, mid-range and preserved ejection fraction. Front Cardiovasc Med 6:125
Tsuchida T, Fukuma N, Oikawa K, Kato K, Kato Y, Takano T, Kumita S (2007) Relationship between plasma norepinephrine at peak exercise and 123I-MIBG imaging of the heart and lower limbs in heart failure. J Nippon Med Sch 74:114–122
Jimenez-Marrero S, Moliner P, Rodríguez-Costoya I, Enjuanes C, Alcoberro L, Yun S, Gonzalez-Costello J, Garay A, Tajes M, Calero E, Hidalgo E, Guerrero C, García-Romero E, Díez-López C, Cainzos-Achirica M, Comin-Colet J (2020) Sympathetic activation and outcomes in chronic heart failure: does the neurohormonal hypothesis apply to mid-range and preserved ejection fraction patients?. Eur J Intern Med 81:60–66
Seravalle G, Quarti-Trevano F, Dell’Oro R, Gronda E, Spaziani D, Facchetti R, Cuspidi C, Mancia G, Grassi G (2019) Sympathetic and baroreflex alterations in congestive heart failure with preserved, midrange and reduced ejection fraction. J Hypertens 37:443–448
Florea VG, Rector TS, Anand IS, Cohn JN (2016) Heart failure with improved ejection fraction: clinical characteristics, correlates of recovery, and survival: results from the valsartan heart failure trial. Circ Heart Fail 9
Keteyian SJ, Brawner CA, Schairer JR, Levine TB, Levine AB, Rogers FJ, Goldstein S (1999) Effects of exercise training on chronotropic incompetence in patients with heart failure. Am Heart J 138:233–240
Awad M, Czer LS, Hou M, Golshani SS, Goltche M, De Robertis M, Kittleson M, Patel J, Azarbal B, Kransdorf E, Esmailian F, Trento A, Kobashigawa JA (2016) Early denervation and later reinnervation of the heart following cardiac transplantation: a review. J Am Heart Assoc 5
Nytrøen K, Gullestad L (2013) Exercise after heart transplantation: an overview. World J Transplant 3:78–90
Guimarães GV, D’Avila V, Bocchi EA, Carvalho VO (2010) Norepinephrine remains increased in the six-minute walking test after heart transplantation. Clinics (Sao Paulo, Brazil) 65:587–591
Braith RW, Wood CE, Limacher MC, Pollock ML, Lowenthal DT, Phillips MI, Staples ED (1992) Abnormal neuroendocrine responses during exercise in heart transplant recipients. Circulation 86:1453–1463
Kitzman DW, Groban L (2008) Exercise intolerance. Heart Fail Clin 4:99–115
Kitzman DW, Groban L (2011) Exercise intolerance. Cardiol Clin 29:461–477
Brubaker PH, Kitzman DW (2011) Chronotropic incompetence: causes, consequences, and management. Circulation 123:1010–1020
Colucci WS, Ribeiro JP, Rocco MB, Quigg RJ, Creager MA, Marsh JD, Gauthier DF, Hartley LH (1989) Impaired chronotropic response to exercise in patients with congestive heart failure. Role of postsynaptic beta-adrenergic desensitization. Circulation 80:314–323
Madamanchi A (2007) Beta-adrenergic receptor signaling in cardiac function and heart failure. McGill J Med: MJM : Int Forum Advance Med Sci Stud 10:99–104
Bristow MR, Hershberger RE, Port JD, Gilbert EM, Sandoval A, Rasmussen R, Cates AE, Feldman AM (1990) Beta-adrenergic pathways in nonfailing and failing human ventricular myocardium. Circulation 82:I12-25
Leclerc KM, Levy WC (2003) The role of norepinephrine in exercise impairment in congestive heart failure. Congest Heart Fail 9:25–28
van Brummelen P, Jie K, van Zwieten PA (1986) Alpha-adrenergic receptors in human blood vessels. Br J Clin Pharmacol 21(Suppl 1):33s–39s
Wilson RA, Di Mario C, Krams R, Soei LK, Wenguang L, Laird AC, The SH, Gussenhoven E, Verdouw P, Roelandt JR (1995) In vivo measurement of regional large artery compliance by intravascular ultrasound under pentobarbital anesthesia. Angiology 46:481–488
Lage SG, Kopel L, Monachini MC, Medeiros CJ, Pileggi F, Polak JF, Creager MA (1994) Carotid arterial compliance in patients with congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol 74:691–695
Francis GS, Goldsmith SR, Cohn JN (1982) Relationship of exercise capacity to resting left ventricular performance and basal plasma norepinephrine levels in patients with congestive heart failure. Am Heart J 104:725–731
Zweerink A, van der Lingen ACJ, Handoko ML, van Rossum AC, Allaart CP (2018) Chronotropic incompetence in chronic heart failure. Circ Heart Fail 11:e004969
De Feo S, Franceschini L, Brighetti G, Cicoira M, Zanolla L, Rossi A, Golia G, Zardini P (2005) Ischemic etiology of heart failure identifies patients with more severely impaired exercise capacity. Int J Cardiol 104:292–297
de Groote P, Soudan B, Lamblin N, Rouaix-Emery N, Mc Fadden E, Meurice T, Mouquet F, Bauters C (2004) Is hormonal activation during exercise useful for risk stratification in patients with moderate congestive heart failure?. Am Heart J 148:349–355
Passino C, Poletti R, Bramanti F, Prontera C, Clerico A, Emdin M (2006) Neuro-hormonal activation predicts ventilatory response to exercise and functional capacity in patients with heart failure. Eur J Heart Fail 8:46–53
Itoh K, Osada N, Inoue K, Samejima H, Seki A, Omiya K, Miyake F (2005) Relationship between exercise intolerance and levels of neurohormonal factors and proinflammatory cytokines in patients with stable chronic heart failure. Int Heart J 46:1049–1059
Passino C, Severino S, Poletti R, Piepoli MF, Mammini C, Clerico A, Gabutti A, Nassi G, Emdin M (2006) Aerobic training decreases B-type natriuretic peptide expression and adrenergic activation in patients with heart failure. J Am Coll Cardiol 47:1835–1839
Leclerc KM, Steele NP, Levy WC (2000) Norepinephrine alters exercise oxygen consumption in heart failure patients. Med Sci Sports Exerc 32:2029–2034
Brunner-La Rocca HP, Weilenmann D, Follath F, Schlumpf M, Rickli H, Schalcher C, Maly FE, Candinas R, Kiowski W (1999) Oxygen uptake kinetics during low level exercise in patients with heart failure: relation to neurohormones, peak oxygen consumption, and clinical findings. Heart 81:121–127
Chandrashekhar Y, Anand IS (1992) Relation between major indices of prognosis in patients with chronic congestive heart failure: studies of maximal exercise oxygen consumption, neurohormones and ventricular function. Indian Heart J 44:213–216
Ogino K, Kato M, Noguchi N, Kitamura H, Osaki S, Omodani H, Matsumoto T, Kinugawa T, Miyakoda H, Kotake H, Mashiba H (1997) Effects of enalapril on the exercise capacity and neurohumoral factors during exercise in patients with chronic heart failure. Cardiology 88:6–13
Lang CC, Rayos GH, Chomsky DB, Wood AJ, Wilson JR (1997) Effect of sympathoinhibition on exercise performance in patients with heart failure. Circulation 96:238–245
Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA (2006) Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 114:2138–2147
Giannoni A, Emdin M, Poletti R, Bramanti F, Prontera C, Piepoli M, Passino C (2008) Clinical significance of chemosensitivity in chronic heart failure: influence on neurohormonal derangement, Cheyne-Stokes respiration and arrhythmias. Clin Sci (Lond) 114:489–497
Benes J, Kotrc M, Borlaug BA, Lefflerova K, Jarolim P, Bendlova B, Jabor A, Kautzner J, Melenovsky V (2013) Resting heart rate and heart rate reserve in advanced heart failure have distinct pathophysiologic correlates and prognostic impact: a prospective pilot study. JACC Heart failure 1:259–266
Kubo T, Parker JD, Azevedo ER, Atchison DJ, Newton GE, Picton P, Floras JS (2005) Vagal heart rate responses to chronic beta-blockade in human heart failure relate to cardiac norepinephrine spillover. Eur J Heart Fail 7:878–881
Santostasi G, Fraccarollo D, Dorigo P, Egloff C, Miraglia G, Marinato PG, Villanova C, Fasoli G, Maragno I (1998) Early reduction in plasma norepinephrine during beta-blocking therapy with metoprolol in chronic heart failure. J Card Fail 4:177–184
Mattioli AV, Mattioli G (1991) Neurohumoral changes in patients with heart failure treated chronically with beta-blockers. Cardiologia (Rome, Italy) 36:549–556
Berg T (2014) β1-blockers lower norepinephrine release by inhibiting presynaptic, facilitating β1-adrenoceptors in normotensive and hypertensive rats. Front Neurol 5:51
Binkley PF, Lewe R, Lima J, Unverferth DV, Leier CV (1988) Neurohumoral profile in congestive heart failure: response to beta-blockade. J Lab Clin Med 111:393–398
Hirsh BJ, Mignatti A, Garan AR, Uriel N, Colombo P, Sims DB, Jorde UP (2012) Effect of β-blocker cessation on chronotropic incompetence and exercise tolerance in patients with advanced heart failure. Circ Heart Fail 5:560–565
Van Thielen G, Paelinck BP, Beckers P, Vrints CJ, Conraads VM (2008) Rate response and cardiac resynchronisation therapy in chronic heart failure: higher cardiac output does not acutely improve exercise performance: a pilot trial. Eur J Cardiovasc Prev Rehabil 15:197–202
Passman R, Banthia S, Galvez D, Sheldon T, Kadish A (2009) The effects of rate-adaptive atrial pacing versus ventricular backup pacing on exercise capacity in patients with left ventricular dysfunction. Pacing Clin Electrophysiol 32:1–6
Tse HF, Siu CW, Lee KL, Fan K, Chan HW, Tang MO, Tsang V, Lee SW, Lau CP (2005) The incremental benefit of rate-adaptive pacing on exercise performance during cardiac resynchronization therapy. J Am Coll Cardiol 46:2292–2297
Palmisano P, Aspromonte V, Ammendola E, Dell’era G, Ziacchi M, Guerra F, Aquilani S, Maglia G, Del Giorno G, Giubertoni A, Boriani G, Capucci A, Pietro Ricci R, Accogli M (2017) Effect of fixed-rate vs. rate-responsive pacing on exercise capacity in patients with permanent, refractory atrial fibrillation and left ventricular dysfunction treated with atrioventricular junction ablation and biventricular pacing (RESPONSIBLE): a prospective, multicentre, randomized, single-blind study. EP Europace 19:414–420
Sims DB, Mignatti A, Colombo PC, Uriel N, Garcia LI, Ehlert FA, Jorde UP (2011) Rate responsive pacing using cardiac resynchronization therapy in patients with chronotropic incompetence and chronic heart failure. Europace 13:1459–1463
Alvarez Villela M, Guerrero-Miranda CY, Chinnadurai T, Patel SR, Jorde UP (2020) Rate response pacing in left ventricular assist device patients. Asaio j 66:e29–e30
Haltern G, Kempa L, Ochs JG, Hanrath P, Sigmund M (1995) Chronic frequency-adaptive pacemaker therapy in patients with heart failure. Z Kardiol 84:834–843
Serova M, Andreev D, Giverts I, Sazonova Y, Svet A, Kuklina M, Sedov V, Syrkin A, Saner H (2020) A new algorithm for optimization of rate-adaptive pacing improves exercise tolerance in patients with HFpEF. Pacing Clin Electrophysiol 43:223–233
Jamil HA, Gierula J, Paton MF, Byrom R, Lowry JE, Cubbon RM, Cairns DA, Kearney MT, Witte KK (2016) Chronotropic incompetence does not limit exercise capacity in chronic heart failure. J Am Coll Cardiol 67:1885–1896
Pieragnoli P, Colella A, Michelucci A, Giusti N, Militello C, Audoglio R, Padeletti L (2003) A new algorithm for closed-loop stimulation: a feasibility study. Pacing Clin Electrophysiol 26:229–232
Cron TA, Pouskoulas CD, Keller DI, Zaugg CE, Buser PT, Pfisterer ME, Osswald S, Hilti P, Schächinger H (2003) Rate response of a closed-loop stimulation pacing system to changing preload and afterload conditions. Pacing Clin Electrophysiol 26:1504–1510
Lindovská M, Kameník L, Pollock B, Hoenen S, Bökelmann T, Spitzer W, Salbach P, Behroz A, Frey A (2012) Clinical observations with closed loop stimulation pacemakers in a large patient cohort: the CYLOS routine documentation registry (RECORD). Europace 14:1587–1595
Chandiramani S, Cohorn LC, Chandiramani S (2007) Heart rate changes during acute mental stress with closed loop stimulation: report on two single-blinded, pacemaker studies. Pacing Clin Electrophysiol 30:976–984
Proff J, Merkely B, Papp R, Lenz C, Nordbeck P, Butter C, Meyerhoefer J, Doering M, MacCarter DJ, Ingel K, Thouet T, Landmesser U, Roser MJ (2021) Impact of closed loop stimulation on prognostic cardiopulmonary variables in patients with chronic heart failure and severe chronotropic incompetence: a pilot, randomized, crossover study. Europace 23:1777–1786
Proietti R, Manzoni G, Di Biase L, Castelnuovo G, Lombardi L, Fundarò C, Vegliante N, Pietrabissa G, Santangeli P, Canby RA, Sagone A, Viecca M, Natale A (2012) Closed loop stimulation is effective in improving heart rate and blood pressure response to mental stress: report of a single-chamber pacemaker study in patients with chronotropic incompetent atrial fibrillation. Pacing Clin Electrophysiol 35:990–998
Coenen M, Malinowski K, Spitzer W, Schuchert A, Schmitz D, Anelli-Monti M, Maier SK, Estlinbaum W, Bauer A, Muehling H, Kalscheur F, Puerner K, Boergel J, Osswald S (2008) Closed loop stimulation and accelerometer-based rate adaptation: results of the PROVIDE study. Europace 10:327–333
Braith RW, Clapp L, Brown T, Brown C, Schofield R, Mills RM, Hill JA (2000) Rate-responsive pacing improves exercise tolerance in heart transplant recipients: a pilot study. J Cardiopulm Rehabil 20:377–382
Author information
Authors and Affiliations
Contributions
Conception: Liza Grosman-Rimon, Erez Kachel Writing the manuscript: Liza Grosman-Rimon, Even Wright, Solomon Sabovich Drafting sections of the manuscript: Even Wright, Solomon Sabovich, Jordan Rimon, Spencer D. Lalonde Critically revising of the manuscript: David Gutterman, Michael A. McDonald, Sagi Gleitman, Doron Sudarsky, Alla Lubovich, Itzhak Gabizon, Sharon Tsuk Preparing figures: Jordan Rimon, David Gutterman Preparing tables: Liza Grosman-Rimon, Even Wright, Solomon Sabovich, Spencer D. Lalonde Approval of the version of the manuscript: Ulrich P. Jorde, Vivek Rao, Shemy Carasso, Erez Kachel
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grosman-Rimon, L., Wright, E., Sabovich, S. et al. Relationships among norepinephrine levels, exercise capacity, and chronotropic responses in heart failure patients. Heart Fail Rev 28, 35–45 (2023). https://doi.org/10.1007/s10741-022-10232-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-022-10232-y