Abstract
Human heart failure (HF) is one of the leading causes of morbidity and mortality worldwide. Currently, heart transplantation and implantation of mechanical devices represent the only available treatments for advanced HF. Two alternative strategies have emerged to treat patients with HF. One approach relies on transplantation of exogenous stem cells (SCs) of non-cardiac or cardiac origin to induce cardiac regeneration and improve ventricular function. Another complementary strategy relies on stimulation of the endogenous regenerative capacity of uninjured cardiac progenitor cells to rebuild cardiac muscle and restore ventricular function. Various SC types and delivery strategies have been examined in the experimental and clinical settings; however, neither the ideal cell type nor the cell delivery method for cardiac cell therapy has yet emerged. Although the use of bone marrow (BM)-derived cells, most frequently exploited in clinical trials, appears to be safe, the results are controversial. Two recent randomized trials have failed to document any beneficial effects of intracardiac delivery of autologous BM mononuclear cells on cardiac function of patients with HF. The remarkable discovery that various populations of cardiac progenitor cells (CPCs) are present in the adult human heart and that it possesses limited regeneration capacity has opened a new era in cardiac repair. Importantly, unlike BM-derived SCs, autologous CPCs from myocardial biopsies cultured and subsequently delivered by coronary injection to patients have given positive results. Although these data are promising, a better understanding of how to control proliferation and differentiation of CPCs, to enhance their recruitment and survival, is required before CPCs become clinically applicable therapeutics.


Similar content being viewed by others
References
Rosamond W et al (2007) Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 115(5):e69–e171
Taylor DA, Zenovich AG (2008) Cardiovascular cell therapy and endogenous repair. Diabetes Obes Metab 10(Suppl 4):5–15
Roger VL et al (2011) Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 123(4):e18–e209
Hoffman JI, Kaplan S (2002) The incidence of congenital heart disease. J Am Coll Cardiol 39(12):1890–1900
Passier R, van Laake LW, Mummery CL (2008) Stem-cell-based therapy and lessons from the heart. Nature 453(7193):322–329
Laflamme MA, Murry CE (2011) Heart regeneration. Nature 473(7347):326–335
Choi WY, Poss KD (2012) Cardiac regeneration. Curr Top Dev Biol 100:319–344
Ptaszek LM et al (2012) Towards regenerative therapy for cardiac disease. Lancet 379(9819):933–942
Hwang H, Kloner RA (2010) Improving regenerating potential of the heart after myocardial infarction: factor-based approach. Life Sci 86(13–14):461–472
Ghadge SK et al (2011) SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther 129(1):97–108
Limana F, Capogrossi MC, Germani A (2011) The epicardium in cardiac repair: from the stem cell view. Pharmacol Ther 129(1):82–96
Martinez EC, Kofidis T (2011) Adult stem cells for cardiac tissue engineering. J Mol Cell Cardiol 50(2):312–319
Zimmermann WH (2011) Embryonic and embryonic-like stem cells in heart muscle engineering. J Mol Cell Cardiol 50(2):320–326
Till JE, McCulloch EA, Siminovitch L (1964) A stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cells. Proc Natl Acad Sci USA 51:29–36
Hagege AA et al (2003) Viability and differentiation of autologous skeletal myoblast grafts in ischaemic cardiomyopathy. Lancet 361(9356):491–492
Menasche P (2007) Skeletal myoblasts as a therapeutic agent. Prog Cardiovasc Dis 50(1):7–17
Olivares EL et al (2004) Bone marrow stromal cells improve cardiac performance in healed infarcted rat hearts. Am J Physiol Heart Circ Physiol 287(2):H464–H470
Xu M et al (2004) Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation 110(17):2658–2665
Murry CE et al (2004) Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 428(6983):664–668
Kao RL, Rizzo C, Magovern GJ (1989) Satellite cells for myocardial regeneration [abstract]. Physiologist 32:220
Taylor DA et al (1998) Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med 4(8):929–933
Menasche P et al (2001) Myoblast transplantation for heart failure. Lancet 357(9252):279–280
Menasche P et al (2008) The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation 117(9):1189–1200
Siminiak T et al (2005) Percutaneous trans-coronary-venous transplantation of autologous skeletal myoblasts in the treatment of post-infarction myocardial contractility impairment: the POZNAN trial. Eur Heart J 26(12):1188–1195
Smits PC et al (2003) Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: clinical experience with six-month follow-up. J Am Coll Cardiol 42(12):2063–2069
Fernandes S et al (2006) Autologous myoblast transplantation after myocardial infarction increases the inducibility of ventricular arrhythmias. Cardiovasc Res 69(2):348–358
Yin AH et al (1997) AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 90(12):5002–5012
Goodell MA et al (1997) Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med 3(12):1337–1345
Sato T, Laver JH, Ogawa M (1999) Reversible expression of CD34 by murine hematopoietic stem cells. Blood 94(8):2548–2554
Tomita S et al (1999) Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 100(19 Suppl):II247–II256
Haider H, Ashraf M (2005) Bone marrow stem cell transplantation for cardiac repair. Am J Physiol Heart Circ Physiol 288(6):H2557–H2567
Pittenger MF et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Ryan JM et al (2005) Mesenchymal stem cells avoid allogeneic rejection. J Inflamm 2:8
Eisenberg LM, Burns L, Eisenberg CA (2003) Hematopoietic cells from bone marrow have the potential to differentiate into cardiomyocytes in vitro. Anat Rec A Discov Mol Cell Evol Biol 274(1):870–882
Alvarez-Dolado M et al (2003) Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425(6961):968–973
Nygren JM et al (2004) Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med 10(5):494–501
Zhang N et al (2006) Blood-borne stem cells differentiate into vascular and cardiac lineages during normal development. Stem Cells Dev 15(1):17–28
Kawada H et al (2004) Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood 104(12):3581–3587
Rota M et al (2007) Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA 104(45):17783–17788
Wollert KC et al (2004) Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364(9429):141–148
Janssens S et al (2006) Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 367(9505):113–121
Lunde K et al (2006) Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med 355(12):1199–1209
Assmus B et al (2010) Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail 3(1):89–96
Strauer BE, Yousef M, Schannwell CM (2010) The acute and long-term effects of intracoronary Stem cell Transplantation in 191 patients with chronic heARt failure: the STAR-heart study. Eur J Heart Fail 12(7):721–729
Zimmet H et al (2012) Short- and long-term outcomes of intracoronary and endogenously mobilized bone marrow stem cells in the treatment of ST-segment elevation myocardial infarction: a meta-analysis of randomized control trials. Eur J Heart Fail 14(1):91–105
Perin EC et al (2012) Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA 307(16):1717–1726
Korf-Klingebiel M et al (2008) Bone marrow cells are a rich source of growth factors and cytokines: implications for cell therapy trials after myocardial infarction. Eur Heart J 29(23):2851–2858
Mirotsou M et al (2011) Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol 50(2):280–289
Gluckman E et al (1989) Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med 321(17):1174–1178
Kogler G et al (2004) A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med 200(2):123–135
Gluckman E (2009) Ten years of cord blood transplantation: from bench to bedside. Br J Haematol 147(2):192–199
Henning RJ et al (2007) Human cord blood cells and myocardial infarction: effect of dose and route of administration on infarct size. Cell Transpl 16(9):907–917
Henning RJ et al (2010) Human umbilical cord blood mononuclear cells decrease fibrosis and increase cardiac function in cardiomyopathy. Regen Med 5(1):45–54
Kehat I et al (2001) Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest 108(3):407–414
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Yamanaka S (2007) Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell 1(1):39–49
Thomson JA et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147
Passier R, Denning C, Mummery C (2006) Cardiomyocytes from human embryonic stem cells. Handb Exp Pharmacol 174:101–122
Orford KW, Scadden DT (2008) Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet 9(2):115–128
Chen L, Daley GQ (2008) Molecular basis of pluripotency. Hum Mol Genet 17(R1):R23–R27
Murry CE, Keller G (2008) Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132(4):661–680
Mummery C et al (2003) Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation 107(21):2733–2740
Laflamme MA et al (2007) Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25(9):1015–1024
van Laake LW et al (2007) Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res 1(1):9–24
Laflamme MA, Murry CE (2005) Regenerating the heart. Nat Biotechnol 23(7):845–856
Liu YP et al (2004) Maintenance of pluripotency in human embryonic stem cells stably over-expressing enhanced green fluorescent protein. Stem Cells Dev 13(6):636–645
Nussbaum J et al (2007) Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J 21(7):1345–1357
Saric T, Frenzel LP, Hescheler J (2008) Immunological barriers to embryonic stem cell-derived therapies. Cells Tissues Organs 188(1–2):78–90
Trounson A et al (2011) Clinical trials for stem cell therapies. BMC Med 9:52
Jaenisch R, Young R (2008) Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132(4):567–582
Takahashi K et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872
Shiba Y, Hauch KD, Laflamme MA (2009) Cardiac applications for human pluripotent stem cells. Curr Pharm Des 15(24):2791–2806
Yu J et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318(5858):1917–1920
Dimos JT et al (2008) Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321(5893):1218–1221
Ebert AD et al (2009) Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457(7227):277–280
Hotta A et al (2009) Isolation of human iPS cells using EOS lentiviral vectors to select for pluripotency. Nat Methods 6(5):370–376
Maehr R et al (2009) Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA 106(37):15768–15773
Chambers I, Smith A (2004) Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene 23(43):7150–7160
Nakagawa M et al (2008) Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 26(1):101–106
Wernig M et al (2008) c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell 2(1):10–12
Hanna J et al (2007) Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 318(5858):1920–1923
Narazaki G et al (2008) Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation 118(5):498–506
Schenke-Layland K et al (2008) Reprogrammed mouse fibroblasts differentiate into cells of the cardiovascular and hematopoietic lineages. Stem Cells 26(6):1537–1546
Kuzmenkin A et al (2009) Functional characterization of cardiomyocytes derived from murine induced pluripotent stem cells in vitro. FASEB J 23(12):4168–4180
Yang L et al (2008) Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453(7194):524–528
Xu XQ et al (2008) Chemically defined medium supporting cardiomyocyte differentiation of human embryonic stem cells. Differentiation 76(9):958–970
Zhu WZ et al (2010) Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res 107(6):776–786
Paige SL et al (2010) Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS ONE 5(6):e11134
Noseda M et al (2011) Cardiopoietic factors: extracellular signals for cardiac lineage commitment. Circ Res 108(1):129–152
Shi Y et al (2008) Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell 3(5):568–574
Ieda M et al (2010) Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142(3):375–386
Efe JA et al (2011) Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol 13(3):215–222
Kim D et al (2009) Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 4(6):472–476
Kaji K et al (2009) Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 458(7239):771–775
Woltjen K et al (2009) piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458(7239):766–770
Yu J et al (2009) Human induced pluripotent stem cells free of vector and transgene sequences. Science 324(5928):797–801
Warren L et al (2010) Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7(5):618–630
Kelly RG, Brown NA, Buckingham ME (2001) The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell 1(3):435–440
Mjaatvedt CH et al (2001) The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol 238(1):97–109
Waldo KL et al (2001) Conotruncal myocardium arises from a secondary heart field. Development 128(16):3179–3188
Kelly RG (2012) The second heart field. Curr Top Dev Biol 100:33–65
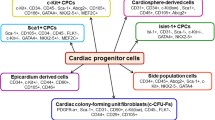
Beltrami AP et al (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114(6):763–776
Linke A et al (2005) Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA 102(25):8966–8971
Urbanek K et al (2006) Stem cell niches in the adult mouse heart. Proc Natl Acad Sci USA 103(24):9226–9231
Bearzi C et al (2007) Human cardiac stem cells. Proc Natl Acad Sci USA 104(35):14068–14073
Bollini S, Smart N, Riley PR (2011) Resident cardiac progenitor cells: at the heart of regeneration. J Mol Cell Cardiol 50(2):296–303
Leri A, Kajstura J, Anversa P (2011) Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res 109(8):941–961
Bernstein HS, Srivastava D (2012) Stem cell therapy for cardiac disease. Pediatr Res 71(4 Pt 2):491–499
Hosoda T et al (2009) Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci USA 106(40):17169–17174
Goodell MA et al (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183(4):1797–1806
Martin CM et al (2004) Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol 265(1):262–275
Pfister O et al (2008) Role of the ATP-binding cassette transporter Abcg2 in the phenotype and function of cardiac side population cells. Circ Res 103(8):825–835
Pfister O et al (2010) Isolation of resident cardiac progenitor cells by Hoechst 33342 staining. Methods Mol Biol 660:53–63
Rasmussen TL et al (2011) Getting to the heart of myocardial stem cells and cell therapy. Circulation 123(16):1771–1779
Pfister O et al (2005) CD31− but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res 97(1):52–61
Wang X et al (2006) The role of the sca-1+/CD31− cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells 24(7):1779–1788
Steinhauser ML, Lee RT (2011) Regeneration of the heart. EMBO Mol Med 3(12):701–712
Lyman SD, Jacobsen SE (1998) c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood 91(4):1101–1134
Dawn B et al (2005) Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci USA 102(10):3766–3771
Miyamoto S et al (2010) Characterization of long-term cultured c-kit+ cardiac stem cells derived from adult rat hearts. Stem Cells Dev 19(1):105–116
Tillmanns J et al (2008) Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci USA 105(5):1668–1673
Tang XL et al (2010) Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation 121(2):293–305
Tallini YN et al (2009) c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci USA 106(6):1808–1813
Zaruba MM et al (2010) Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation 121(18):1992–2000
Bolli R et al (2011) Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378(9806):1847–1857
Holmes C, Stanford WL (2007) Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells 25(6):1339–1347
Matsuura K et al (2004) Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem 279(12):11384–11391
Matsuura K et al (2009) Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest 119(8):2204–2217
Oh H et al (2003) Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA 100(21):12313–12318
Cai CL et al (2003) Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell 5(6):877–889
Laugwitz KL et al (2005) Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 433(7026):647–653
Moretti A et al (2006) Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 127(6):1151–1165
Moretti A et al (2010) Mouse and human induced pluripotent stem cells as a source for multipotent Isl1+ cardiovascular progenitors. FASEB J 24(3):700–711
Messina E et al (2004) Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 95(9):911–921
Smith RR et al (2007) Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115(7):896–908
Chimenti I et al (2010) Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res 106(5):971–980
Bartosh TJ et al (2008) 3D-model of adult cardiac stem cells promotes cardiac differentiation and resistance to oxidative stress. J Cell Biochem 105(2):612–623
Nelson TJ et al (2009) Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation 120(5):408–416
Johnston PV, et al (2009) Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation 120(12):1075–1083, 7 p following 1083
Lee ST et al (2011) Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol 57(4):455–465
Andersen DC et al (2009) Murine “cardiospheres” are not a source of stem cells with cardiomyogenic potential. Stem Cells 27(7):1571–1581
Davis DR et al (2009) Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS ONE 4(9):e7195
Makkar RR et al (2012) Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379(9819):895–904
Siu CW, Tse HF (2012) Cardiac regeneration: messages from CADUCEUS. Lancet 379(9819):870–871
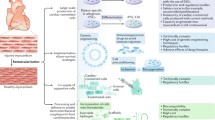
Ishii Y et al (2010) BMP signals promote proepicardial protrusion necessary for recruitment of coronary vessel and epicardial progenitors to the heart. Dev Cell 19(2):307–316
Merki E et al (2005) Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci USA 102(51):18455–18460
Limana F et al (2007) Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res 101(12):1255–1265
van Tuyn J et al (2007) Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells 25(2):271–278
Cai CL et al (2008) A myocardial lineage derives from Tbx18 epicardial cells. Nature 454(7200):104–108
Zhou B et al (2008) Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454(7200):109–113
Red-Horse K et al (2010) Coronary arteries form by developmental reprogramming of venous cells. Nature 464(7288):549–553
Smart N et al (2007) Thymosin beta-4 is essential for coronary vessel development and promotes neovascularization via adult epicardium. Ann N Y Acad Sci 1112:171–188
Bock-Marquette I et al (2009) Thymosin beta4 mediated PKC activation is essential to initiate the embryonic coronary developmental program and epicardial progenitor cell activation in adult mice in vivo. J Mol Cell Cardiol 46(5):728–738
Limana F et al (2010) Myocardial infarction induces embryonic reprogramming of epicardial c-kit(+) cells: role of the pericardial fluid. J Mol Cell Cardiol 48(4):609–618
Smart N et al (2010) Thymosin beta4 facilitates epicardial neovascularization of the injured adult heart. Ann N Y Acad Sci 1194:97–104
Smart N et al (2011) De novo cardiomyocytes from within the activated adult heart after injury. Nature 474(7353):640–644
Zhou B et al (2011) Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest 121(5):1894–1904
Smart N et al (2007) Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature 445(7124):177–182
Ott HC et al (2007) The adult human heart as a source for stem cells: repair strategies with embryonic-like progenitor cells. Nat Clin Pract Cardiovasc Med 4(Suppl 1):S27–S39
Li RK, et al (1996) Cardiomyocyte transplantation improves heart function. Ann Thorac Surg 62(3):654–660; discussion 660–1
Scorsin M, et al (1997) Does transplantation of cardiomyocytes improve function of infarcted myocardium? Circulation 96(9 Suppl):II-188–II-193
Tsonis PA (1996) Limb Regeneration. Cambridge University Press, Cambridge
Rumyantsev PP (1973) Post-injury DNA synthesis, mitosis and ultrastructural reorganization of adult frog cardiac myocytes. An electron microscopic-autoradiographic study. Z Zellforsch Mikrosk Anat 139(3):431–450
Rumyantsev PP (1977) Interrelations of the proliferation and differentiation processes during cardiac myogenesis and regeneration. Int Rev Cytol 51:186–273
Oberpriller JO, Oberpriller JC (1974) Response of the adult newt ventricle to injury. J Exp Zool 187(2):249–253
Bader D, Oberpriller JO (1978) Repair and reorganization of minced cardiac muscle in the adult newt (Notophthalmus viridescens). J Morphol 155(3):349–357
Bader D, Oberpriller J (1979) Autoradiographic and electron microscopic studies of minced cardiac muscle regeneration in the adult newt, Notophthalmus viridescens. J Exp Zool 208(2):177–193
Oberpriller JO et al (1995) Stimulation of proliferative events in the adult amphibian cardiac myocyte. Ann N Y Acad Sci 752:30–46
Poss KD (2007) Getting to the heart of regeneration in zebrafish. Semin Cell Dev Biol 18(1):36–45
Poss KD (2010) Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat Rev Genet 11(10):710–722
Poss KD, Wilson LG, Keating MT (2002) Heart regeneration in zebrafish. Science 298(5601):2188–2190
Lepilina A et al (2006) A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 127(3):607–619
Kikuchi K et al (2010) Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature 464(7288):601–605
Jopling C et al (2010) Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 464(7288):606–609
Chablais F et al (2011) The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev Biol 11:21
Gonzalez-Rosa JM et al (2011) Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 138(9):1663–1674
Schnabel K et al (2011) Regeneration of cryoinjury induced necrotic heart lesions in zebrafish is associated with epicardial activation and cardiomyocyte proliferation. PLoS ONE 6(4):e18503
Wang J et al (2011) The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development 138(16):3421–3430
Li F et al (1996) Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol 28(8):1737–1746
Adler CP (1975) Relationship between deoxyribonucleic acid content and nucleoli in human heart muscle cells and estimation of cell number during cardiac growth and hyperfunction. Recent Adv Stud Cardiac Struct Metab 8:373–386
Bergmann O et al (2009) Evidence for cardiomyocyte renewal in humans. Science 324(5923):98–102
Drenckhahn JD et al (2008) Compensatory growth of healthy cardiac cells in the presence of diseased cells restores tissue homeostasis during heart development. Dev Cell 15(4):521–533
Porrello ER et al (2011) Transient regenerative potential of the neonatal mouse heart. Science 331(6020):1078–1080
Bergmann O et al (2011) Identification of cardiomyocyte nuclei and assessment of ploidy for the analysis of cell turnover. Exp Cell Res 317(2):188–194
Kajstura J et al (2010) Cardiomyogenesis in the adult human heart. Circ Res 107(2):305–315
Dyer LA, Kirby ML (2009) The role of secondary heart field in cardiac development. Dev Biol 336(2):137–144
Rochais F, Mesbah K, Kelly RG (2009) Signaling pathways controlling second heart field development. Circ Res 104(8):933–942
Vincent SD, Buckingham ME (2010) How to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol 90:1–41
Bruneau BG (2002) Transcriptional regulation of vertebrate cardiac morphogenesis. Circ Res 90(5):509–519
Evans SM et al (2010) Myocardial lineage development. Circ Res 107(12):1428–1444
van Weerd JH et al (2011) Epigenetic factors and cardiac development. Cardiovasc Res 91(2):203–211
Behfar A et al (2002) Stem cell differentiation requires a paracrine pathway in the heart. FASEB J 16(12):1558–1566
Yuasa S et al (2005) Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol 23(5):607–611
Naito AT et al (2006) Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci USA 103(52):19812–19817
Kwon C et al (2007) Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci USA 104(26):10894–10899
Hao J et al (2008) Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS ONE 3(8):e2904
Brennan J et al (2001) Nodal signalling in the epiblast patterns the early mouse embryo. Nature 411(6840):965–969
Schultheiss TM, Burch JB, Lassar AB (1997) A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev 11(4):451–462
Foley AC, Mercola M (2005) Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev 19(3):387–396
Ilagan R et al (2006) Fgf8 is required for anterior heart field development. Development 133(12):2435–2445
Park EJ et al (2006) Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development 133(12):2419–2433
Park EJ et al (2008) An FGF autocrine loop initiated in second heart field mesoderm regulates morphogenesis at the arterial pole of the heart. Development 135(21):3599–3610
Urness LD et al (2011) Redundant and dosage sensitive requirements for Fgf3 and Fgf10 in cardiovascular development. Dev Biol 356(2):383–397
High FA, Epstein JA (2008) The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet 9(1):49–61
High FA et al (2009) Murine Jagged1/Notch signaling in the second heart field orchestrates Fgf8 expression and tissue-tissue interactions during outflow tract development. J Clin Invest 119(7):1986–1996
Kwon C et al (2009) A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol 11(8):951–957
Hutson MR et al (2010) Arterial pole progenitors interpret opposing FGF/BMP signals to proliferate or differentiate. Development 137(18):3001–3011
Tirosh-Finkel L et al (2010) BMP-mediated inhibition of FGF signaling promotes cardiomyocyte differentiation of anterior heart field progenitors. Development 137(18):2989–3000
Thomas NA et al (2008) Hedgehog signaling plays a cell-autonomous role in maximizing cardiac developmental potential. Development 135(22):3789–3799
Dyer LA, Kirby ML (2009) Sonic hedgehog maintains proliferation in secondary heart field progenitors and is required for normal arterial pole formation. Dev Biol 330(2):305–317
Zhang XM, Ramalho-Santos M, McMahon AP (2001) Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell 105(6):781–792
Lavine KJ, Kovacs A, Ornitz DM (2008) Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J Clin Invest 118(7):2404–2414
Hoffmann AD et al (2009) sonic hedgehog is required in pulmonary endoderm for atrial septation. Development 136(10):1761–1770
Ryckebusch L et al (2008) Retinoic acid deficiency alters second heart field formation. Proc Natl Acad Sci USA 105(8):2913–2918
Sirbu IO, Zhao X, Duester G (2008) Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev Dyn 237(6):1627–1635
Li P, Pashmforoush M, Sucov HM (2010) Retinoic acid regulates differentiation of the secondary heart field and TGFbeta-mediated outflow tract septation. Dev Cell 18(3):480–485
Schleiffarth JR et al (2007) Wnt5a is required for cardiac outflow tract septation in mice. Pediatr Res 61(4):386–391
Zhou W et al (2007) Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat Genet 39(10):1225–1234
Zhou Y et al (2011) Latent TGF-beta binding protein 3 identifies a second heart field in zebrafish. Nature 474(7353):645–648
Olivetti G et al (1991) Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res 68(6):1560–1568
Olivetti G et al (1997) Apoptosis in the failing human heart. N Engl J Med 336(16):1131–1141
Guerra S et al (1999) Myocyte death in the failing human heart is gender dependent. Circ Res 85(9):856–866
Murry CE, Reinecke H, Pabon LM (2006) Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol 47(9):1777–1785
Itzhaki-Alfia A et al (2009) Patient characteristics and cell source determine the number of isolated human cardiac progenitor cells. Circulation 120(25):2559–2566
Lien CL et al (2006) Gene expression analysis of zebrafish heart regeneration. PLoS Biol 4(8):e260
Engel FB et al (2005) p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev 19(10):1175–1187
Engel FB et al (2006) FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci USA 103(42):15546–15551
Boni A et al (2008) Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci USA 105(40):15529–15534
Croquelois A et al (2008) Control of the adaptive response of the heart to stress via the Notch1 receptor pathway. J Exp Med 205(13):3173–3185
Collesi C et al (2008) Notch1 signaling stimulates proliferation of immature cardiomyocytes. J Cell Biol 183(1):117–128
Koyanagi M et al (2007) Notch signaling contributes to the expression of cardiac markers in human circulating progenitor cells. Circ Res 101(11):1139–1145
Rosenblatt-Velin N et al (2005) FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Invest 115(7):1724–1733
Urbanek K et al (2005) Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res 97(7):663–673
Rota M et al (2008) Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res 103(1):107–116
Aghila Rani KG, Kartha CC (2010) Effects of epidermal growth factor on proliferation and migration of cardiosphere-derived cells expanded from adult human heart. Growth Factors 28(3):157–165
Gonzalez A et al (2008) Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res 102(5):597–606
Gude NA et al (2008) Activation of Notch-mediated protective signaling in the myocardium. Circ Res 102(9):1025–1035
Sun Y (2010) Intracardiac renin-angiotensin system and myocardial repair/remodeling following infarction. J Mol Cell Cardiol 48(3):483–489
Segers VF, Lee RT (2010) Protein therapeutics for cardiac regeneration after myocardial infarction. J Cardiovasc Transl Res 3(5):469–477
Bocchi L et al (2011) Growth factor-induced mobilization of cardiac progenitor cells reduces the risk of arrhythmias, in a rat model of chronic myocardial infarction. PLoS ONE 6(3):e17750
Pesce M et al (2011) Endothelial and cardiac progenitors: boosting, conditioning and (re)programming for cardiovascular repair. Pharmacol Ther 129(1):50–61
Hoover-Plow J, Gong Y (2012) Challenges for heart disease stem cell therapy. Vasc Health Risk Manag 8:99–113
Bock-Marquette I et al (2004) Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 432(7016):466–472
Srivastava D et al (2007) Thymosin beta4 is cardioprotective after myocardial infarction. Ann N Y Acad Sci 1112:161–170
Takahashi K et al (2008) Modulated inflammation by injection of high-mobility group box 1 recovers post-infarction chronically failing heart. Circulation 118(14 Suppl):S106–S114
Kohno T et al (2009) Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc Res 81(3):565–573
Palumbo R, Bianchi ME (2004) High mobility group box 1 protein, a cue for stem cell recruitment. Biochem Pharmacol 68(6):1165–1170
Palumbo R et al (2004) Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol 164(3):441–449
Limana F et al (2005) Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res 97(8):e73–e83
Hofmann M et al (2005) Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation 111(17):2198–2202
Hou D et al (2005) Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation 112(9 Suppl):I150–I156
Perin EC, Lopez J (2006) Methods of stem cell delivery in cardiac diseases. Nat Clin Pract Cardiovasc Med 3(Suppl 1):S110–S113
Beeres SL et al (2008) Cell therapy for ischaemic heart disease. Heart 94(9):1214–1226
Martin-Rendon E et al (2008) Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J 29(15):1807–1818
Freyman T et al (2006) A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J 27(9):1114–1122
Kurpisz M et al (2007) Bone marrow stem cell imaging after intracoronary administration. Int J Cardiol 121(2):194–195
Hare JM et al (2009) A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 54(24):2277–2286
Aicher A et al (2003) Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation 107(16):2134–2139
Blocklet D et al (2006) Myocardial homing of nonmobilized peripheral-blood CD34+ cells after intracoronary injection. Stem Cells 24(2):333–336
Robey TE et al (2008) Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol 45(4):567–581
Muller-Ehmsen J et al (2002) Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol 34(2):107–116
Zeng L et al (2007) Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation 115(14):1866–1875
Hansson EM, Lindsay ME, Chien KR (2009) Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell 5(4):364–377
Rangappa S, Makkar R, Forrester J (2010) Review article: current status of myocardial regeneration: new cell sources and new strategies. J Cardiovasc Pharmacol Ther 15(4):338–343
Malliaras K, Marban E (2011) Cardiac cell therapy: where we’ve been, where we are, and where we should be headed. Br Med Bull 98:161–185
Martens TP et al (2009) Percutaneous cell delivery into the heart using hydrogels polymerizing in situ. Cell Transpl 18(3):297–304
Hamdi H et al (2009) Cell delivery: intramyocardial injections or epicardial deposition? A head-to-head comparison. Ann Thorac Surg 87(4):1196–1203
Miyagawa S et al (2009) Combined autologous cellular cardiomyoplasty using skeletal myoblasts and bone marrow cells for human ischemic cardiomyopathy with left ventricular assist system implantation: report of a case. Surg Today 39(2):133–136
Miyagawa S et al (2011) Tissue-engineered cardiac constructs for cardiac repair. Ann Thorac Surg 91(1):320–329
Furuta A et al (2006) Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ Res 98(5):705–712
Domian IJ et al (2009) Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science 326(5951):426–429
Pasha Z et al (2008) Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res 77(1):134–142
Hu X et al (2008) Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg 135(4):799–808
Haider H et al (2008) IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res 103(11):1300–1308
Traverse JH et al (2011) Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA 306(19):2110–2119
Haberland M, Montgomery RL, Olson EN (2009) The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10(1):32–42
Cordes KR, Srivastava D (2009) MicroRNA regulation of cardiovascular development. Circ Res 104(6):724–732
Jakob P, Landmesser U (2012) Role of microRNAs in stem/progenitor cells and cardiovascular repair. Cardiovasc Res 93(4):614–622
Conflict of interest
This manuscript entitled “Myocardial Regeneration of the Failing Heart” by A. T. Akhmedov and J. Marín-García which we are submitting for publication in Heart Failure Reviews has not been, nor will be published elsewhere, has been read and is submitted with the approval of all authors, all of which participated in the writing of the manuscript with no conflict of interest in its publication in Heart Failure Reviews.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akhmedov, A.T., Marín-García, J. Myocardial regeneration of the failing heart. Heart Fail Rev 18, 815–833 (2013). https://doi.org/10.1007/s10741-012-9348-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-012-9348-5