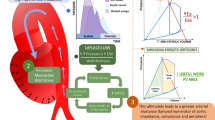
Abstract
Abnormal electrical activation of the ventricles creates major abnormalities in cardiac mechanics. Local contraction patterns, as reflected by measurements of local strain, are not only out of phase, but often also show opposing length changes in early and late activated regions. As a consequence, the efficiency of cardiac pump function (the amount of stroke work generated by a unit of oxygen consumed) is approximately 30% lower in asynchronous than in synchronous hearts. Moreover, the amount of work performed in myocardial segments becomes considerably larger in late than in early activated regions. Cardiac Resynchronization Therapy (CRT) improves mechano-energetics of the previously asynchronous heart in various ways: it alleviates impediment of the abnormal contraction on blood flow, it increases myocardial efficiency, it recruits contraction in the previously early activated septum and it creates a more uniform distribution of myocardial blood flow. These factors act together to increase the range of cardiac work that can be delivered by the patients’ heart, an effect that can explain the increased exercise tolerance and quality of life reported in several CRT trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Until approximately a decade ago, the mechanical effects of asynchronous electrical activation were almost completely neglected, despite the first evidence for detrimental mechanical effects being published 80 years ago by Wiggers [56]. Nowadays, interest on this issue increases rapidly, related to accumulating evidence of beneficial effects of Cardiac Resynchronization Therapy (CRT) [12, 43] and adverse effects of conventional RV pacing [2, 46, 57].
For proper understanding of the effect of ventricular pacing and CRT, insights into cardiac mechanics and energetics are imperative. After all, mechanical coordination between all muscle fibers determines total pump function as well as the amount of energy required for the contraction. Fortunately, information on cardiac mechanics is rapidly increasing due to the development of dedicated non-invasive imaging techniques for assessment of regional myocardial deformation (strains). Also, modern modalities like PET allow achieving information on cardiac metabolism. While in many studies the sole emphasis is on improving pump function, it may also be important to pay attention to the metabolic economy of the heart. After all, clinical studies have shown the potentially harmful effect of longer lasting increase of myocardial energy expenditure through inotropic drug therapy [35].
In this article, we will discuss cardiac mechanics and energetics during asynchronous and resynchronized activation. To this purpose, we review information from studies in animal experimental models and patients. However, not all concepts and ideas have been verified in large clinical trials.
Electrical activation and electro-mechanical coupling
In the healthy heart, activation of the ventricular wall occurs by impulse conduction through the rapid conduction system. Endings of this system are located in the subendocardium. From there, the impulses are conducted through the slower conducting working myocardium. Accordingly, during normal sinus rhythm in hearts without conduction abnormalities the electrical activation is relatively synchronous (activation of the ventricles occurring within 70 ms). Earliest activation occurs in the LV septal endocardium and latest in the epicardium of the LV lateral wall [19]. This is also depicted in the left upper panel of Fig. 1, derived from measurements of epicardial and endocardial mapping in a canine heart [30].
Three dimensional representation of ventricular electrical activation (upper panels) and strain tracings (lower panels) during normal activation (left panels) and LBBB (right panels) in a canine heart. In the LBBB heart, early activation in the septum (blue circles in right upper panel) leads to early onset of shortening (corresponding blue tracing in lower right panel) while late activated LV lateral wall regions (red tracing) are stretched during early systole, followed by pronounced shortening during the ejection phase. Gray tracings represent mean LV strain. Derived from data presented in [30] and [13]
The normal, physiological sequence of electrical activation is disturbed during ventricular pacing [29, 51] as well as in diseases affecting the ventricular conduction system, like left bundle branch block (LBBB) [3, 41, 50]. Under all these circumstances, the impulse is conducted through the slowly conducting working myocardium, rather than through the rapidly conducting specialized conduction system. As a consequence, the time required for activation of the entire ventricular muscle, for example expressed as QRS duration, is at least twice as long as that during normal sinus rhythm. QRS prolongation by abnormal activation is even greater in patients with ischemic heart disease [51].
Like in any muscle, cardiac contraction is evoked by an action potential. The action potential triggers calcium influx through L-type calcium channels, initiating calcium induced calcium release by the sarcoplasmic reticulum (SR). The time needed for transport and binding of calcium, released from the SR, gives rise to a time delay between the depolarization and onset of force development of approximately 30 ms. On a global basis, this delay can be observed as the delay between the R-wave of the ECG and the rise in LV pressure.
Because of this excitation–contraction coupling, it is not surprising that asynchronous electrical activation also leads to asynchronous contraction. In various studies in normal canine heart, the onset of segment shortening proved to be closely related to the timing of electrical activation [5, 37, 59]. Accordingly, regions with earliest electrical activation (blue ellipse in right upper panel of Fig. 1) also start to contract first (blue tracing in lower right panel of Fig. 1).
In order to achieve information on local excitation–contraction coupling, the delay between local electrical activation and strain was measured, often defined as the electro-mechanical (E-M) delay. In normal hearts, this E-M delay amounts approximately 30 ms [37]. With more advanced measurements (MRI tagging) at many sites and in asynchronous ventricles, a linear relation is found between local activation (X) and shortening (Y). The regression line of this relation has an intercept of approximately 20 ms and a slope larger than one [59], indicating that timing differences in shortening are larger than in electrical activation. This larger mechanical asynchrony is presumably explained completely by its definition: the onset of shortening. Recent studies in canine hearts indicate that when the onset of active force generation rather than onset of shortening is used to define mechanical activation, E-M delay is equal throughout the asynchronous heart and directly reflects the timing of electrical activation [42]. This discrepancy between onset of active force generation and onset of shortening is explained by the fact that early activated regions can start to shorten immediately when they are activated, because cavity pressure is low and all other muscle fibers are passive. However, when late-activated regions become activated, other fibers have already started to develop force, thus also increasing cavity pressure. Accordingly, late activated fibers can only start to shorten after developing more force, a process which takes time.
As a consequence of the mechanical interaction between the various regions in the asynchronous heart, strain signals in early and late activated regions do not only show a shift in time, but have a completely different shape (Fig. 1). This implies that asynchronous activation leads to discoordination of contraction. The discoordination is primarily illustrated by early systolic shortening in early activated regions and coincident early systolic stretch in late activated regions. Later in systole, late activated regions show pronounced shortening, while early activated regions may be even stretched [17, 38, 40].
The complicated regional differences in contraction pattern are most likely related to the local differences in myocardial fiber length during the early systolic phase. Fiber length is presumably closely related to sarcomere length, an important determinant of contractile force [47]. In studies using two isolated papillary muscles in series asynchronous stimulation caused a downward shift in the force–velocity relation in the early activated muscle and an upward shift in the late activated one [48]. Therefore, the regional differences in contraction pattern during ventricular pacing are most likely caused by regional differences in effective preload. Accordingly, during ventricular pacing, regional systolic fiber shortening increases with increasing isovolumic stretch [39]. Similarly, a close correlation exists between the time of local electrical activation and the degree of systolic fiber shortening [18] (Fig. 2).
Plot of local subepicardial fiber strain during the ejection phase versus local electrical activation time during four modes of pacing: right atrial (normal activation, filled squares), LV free wall (open squares), LV apex (+) and RV outflow tract pacing (triangles). The activation time was normalized to the moment of maximum rate of rise of LV pressure. A strain value of −0.10 represents 10% shortening. Modified after [17]
Effect of asynchrony on cardiac energetics
Global left ventricular pump work, generated by the summed action of the myocardial fibers, can be derived from left ventricular pressure–volume loops. Total mechanical work, e.g. external mechanical work and the so-called potential mechanical work, is defined as the pressure–volume area (PVA) which is the area bounded by the pressure–volume trajectory during systole and both the end-systolic and end-diastolic pressure–volume relationship curves [44]. Local mechanical work can be derived from local fiber stress-fiber strain loops (Fig. 3). It is conceivable that local differences in wall motion and deformation during asynchronous electrical activation will be reflected in local differences in myocardial work. This was demonstrated in dogs with epicardial pacing at different sites by construction of local fiber stress-fiber strain diagrams and calculation of local external and total mechanical work analogous to the PVA concept used for the entire LV [17, 40]. Direct measurement of fiber stress using a device damages the local myocardial structure and thereby makes this measurement unreliable. Therefore, fiber stress was estimated using data on LV pressure, LV wall volume, LV cavity volume and regional fiber strain [17, 40]. In regions close to the pacing site, the area of the fiber stress-fiber strain loops is small or even negative, indicating low external work or work being performed on that particular region. In regions remote from the pacing site, the loops are wide and external work can be up to twice that during synchronous ventricular activation (Fig. 3). Total myocardial work (sum of external work and potential energy) is reduced by 50% in early activated regions and is increased by 50% in late activated regions, when compared with the situation during atrial pacing [17, 40].
Definition of SRS and ISF, using strain signals from septum and LV lateral wall from a dg with LBBB (same signals as in Fig. 1). The gray part of the curves indicate the diastolic phase, not used for calculation of ISF and SRS
In light of the considerable mechanical differences between regions, it is not surprising that during asynchronous activation also myocardial blood flow [17, 33, 38, 49, 55], oxygen consumption [17, 28] and glucose uptake differs between regions [33]. When compared to sinus rhythm, myocardial blood flow and oxygen consumption are 30% lower in early activated regions and 30% higher in late activated regions [17, 38, 49, 54]. Interestingly, in asynchronous hearts, regional differences in glucose uptake are larger than the differences in blood flow, suggesting a metabolic adaptation beside the adaptation in perfusion [33].
There are various arguments for the idea that the regional differences in blood flow during ventricular pacing are a physiological adaptation to the differences in mechanical work. A good correlation was found between local mechanical work and oxygen consumption during ventricular pacing [17]. These regional differences disappear during total coronary vasodilation by adenosine [1]. This shows that coronary perfusion is not hampered by abnormal contraction and relaxation patterns. Another argument in favor of matching of demand and supply during asynchronous activation is the fact that septal blood flow after chronic experimental LBBB was reduced without signs of hibernation [55]. Only at higher heart rates, when myocardial perfusion becomes more critical, impairment of blood flow due to the abnormal conduction may play a role [7].
Indices of dyssynchrony and discoordination
In this context, distinction is made between dyssynchrony and discoordination. The term dyssynchrony is commonly used for timing of contraction, as expressed by the timing of onset or peak shortening. Discoordination can be defined as a condition of opposing shortening and stretch within the ventricle. In another article in this issue, it is discussed that measurement of strain provides considerably more reliable prediction of response to CRT than measurement of tissue velocity [27]. However, even better prediction of CRT response is provided by indices that reflect discoordination of contraction. Examples are the circumferential uniformity ratio estimate (CURE) [8], Internal Stretch Fraction (ISF) [23] and Septal Rebound Stretch (SRS) [14]. The CURE index is calculated using Fourier analysis of the strains around the LV circumference as the ratio of the sum of the synchronous segments (S0) and the sum of both dyssynchronous (S1) and synchronous segments over a certain period of the cardiac cycle (such as systole). Calculation of ISF and SRS is depicted in Fig. 4. All these indices quantify the amount of stretch during systole. While the SRS quantifies only the stretch in the septum, the other two relate this stretch to shortening in other regions. All three indices were shown to provide excellent predictions of CRT response in single center studies [8, 14, 23]. Moreover, for ISF and SRS, it was also shown that these variables were reduced to practically zero by application of CRT [14]. In a study using a mathematical model, it was demonstrated that ISF and CURE increase due to asynchronous electrical activation as well as due to dilatation, whereas the timing difference in peak shortening decreased during dilatation [22]. This interaction between dilatation and asynchrony may explain the better prediction of CRT response by the measures of discoordination when compared to the measures of dyssynchrony. A physiological consideration is that these indices quantify the amount of wall deformation that opposes ejection of blood and reflect the absorption of energy generated by other regions. Inherently, this implies that these indices reflect the (in)efficiency of contraction.
Bulls-eye plots of the distribution of external myocardial work in the canine left ventricle during normal impulse conduction (atrial pacing) and during asynchronous activation, as induced by RV apex and LV free wall pacing. Simplified fiber stress–fiber length (S–L) relations in regions with normal (red) early (black) and late (yellow) activation are presented. Strain was measured using MRI tagging; stress was estimated from LV pressure and cavity volume and local strain. [17] External work is determined as the area of the S–L loop [40]
Ventricular efficiency
In agreement with the abovementioned thoughts, asynchronous ventricles require more oxygen to generate the same amount of mechanical work, i.e. they have a lower efficiency. In isolated, isovolumically beating hearts, ventricular pacing reduces oxygen consumption and developed LV pressure to the same amount, so that efficiency is not influenced [11]. In anesthetized open-chest [6] and in conscious dogs [34], RV apex pacing decreased mechanical output, whereas myocardial oxygen consumption was unchanged or even increased, when compared to atrial pacing. As a consequence, efficiency decreased by 20–30% in these studies. A similar number has recently been reported by Mills et al., who determined mechanical energy using potential energy and stroke work from pressure volume relations and oxygen consumption by direct measurements [30]. These investigators found a ~30% reduction in efficiency during pacing at the RV apex and RV septum, primarily caused by a reduction in stroke work and increase in oxygen consumption (Fig. 5). This reduction in efficiency was observed briefly after starting RV pacing, but also after 4 months of ventricular pacing, showing the lack of potential of the heart to adapt to this inefficiency. Interestingly, these investigators also showed that pacing at other single sites (LV apex, LV septum) as well as at RV + LV was able to avoid or correct the decrease in efficiency [30]. In the same study, the ~ 30% decrease in efficiency coincided with ISF values of about 30%, suggesting that ISF may provide a mechanical estimation of ventricular efficiency.
Upper panel: Conversion of aerobic energy into stroke work and other forms of energy. Oxygen is used for aerobic metabolism resulting in ATP formation, yet in that step a certain part of the energy is lost as non-mechanical consumption. ATP is used for basal metabolism and calcium handling, still processes that do not lead to mechanical output. Contraction, energized by ATP, leads to a certain area of the LV pressure–volume loop (PVA), of which only stroke work is useful mechanical output for the body. All other energy is ultimately converted into heat. Lower panel: efficiency of conversion of oxygen into mechanical energy in dogs during normal conduction as well as during RV apex and LV apex pacing. Data derived from [30]. RV apex pacing, but not LV apex pacing, decreases the amount of stroke work achieved from a unit volume of oxygen uptake. The lower efficiency during RV pacing is associated with an increased Internal Stretch Fraction (ISF), as indicated by the numbers at the right side of the figure. PVA = pressure volume area (see text). The data provide evidence for the fact that dyssynchrony reduces cardiac efficiency by turning contractile energy into heat, presumably due to stretching of some regions by (stronger) contraction of others
Effects of CRT
Electrical and mechanical resynchronization
CRT is predominantly employed to resynchronize ventricles with LBBB or with RV pacing. In these ventricles, activation spreads from the right ventricular wall through the interventricular septum and subsequently to the LV lateral wall. Conceptually, resynchronization can be achieved by creating more than one wavefront of activation. The most common approach is to use biventricular pacing to create two activation wave fronts, preferably originating from opposite walls, in order to create a more synchronous activation than during RV pacing or LBBB. With the tight excitation–contraction coupling in the heart, the more synchronous activation is expected and indeed creates also a more synchronous and more coordinated contraction (Fig. 6). A similar, and sometimes better, effect can be achieved when using LV pacing at an AV-delay that allows fusion of the wavefront originating from the RBB and from the LV pacing site [52].
Strain renderings of the LV created by mapping myocardial circumferential strain (Ecc) as color on a volume reconstruction of the LV. Blue indicates contraction (negative Ecc), red indicates the reference state (Ecc = 0), and yellow indicates stretch (positive Ecc). Data are shown for late diastole, early systole, midsystole and end systole (sequential columns from left to right). Data obtained using MRI tagging during RA, BiV RV apex (RVa) and LV later wall (LV) pacing, from top to bottom. Black dot next to each heart indicates the location of the midseptum. Modified after [58]
Figure 6 also shows that in the absence of conduction disturbances, the pattern of contraction during biventricular pacing is less uniform than during right atrial pacing, an abnormality that is accompanied by slightly depressed hemodynamics during biventricular pacing [36, 58]. These data are in agreement with observations in the Pacing Therapies in Congestive Heart Failure (PATH-CHF) trial, which showed that in patients without ventricular dyssynchrony LV pump function does not improve by biventricular pacing, but rather worsens upon starting biventricular pacing [4]. Therefore, while biventricular pacing clearly resynchronizes asynchronous hearts, it may worsen the synchrony and sequence of activation in hearts without conduction block. A recent study in CRT patients showed that in so-called non-responders long-term CRT even leads to worsening of echocardiographic parameters rather than that it did not change them [62]. Similarly, recently presented preliminary data from the MADIT-CRT trial indicate that CRT significantly reduces the combined endpoint hospitalization for heart failure and mortality in patients with LBBB. In contrast, patients with equally wide QRS complex, but with diffuse intraventricular conduction disturbances (so no interventricular asynchrony) proved to encounter a 40% increase in the incidence of heart failure or death [61]. Therefore, the decision to start CRT in a patient should be taken with great care, since unjustified application of CRT may worsen the patients’ condition.
Functional improvements by CRT
Many studies have shown the improvement of parameters of cardiac pump function, such as LVdP/dtmax, pulse pressure, cardiac output and ejection fraction [4, 9, 16], [21] [26, 60]. Such improved systolic pump function is achieved at unchanged or even decreased filling pressures, denoting a true improvement of ventricular contractility through improved coordination of contraction [24]. This is also indicated by a leftward shift of the end-systolic pressure–volume point during CRT [16, 21].
Recent measurements of myocardial strain in patients before and during CRT provide better insight into the mechanism of CRT. DeBoeck et al. [14] demonstrated that CRT does not change the total amount of systolic deformation, but that it redistributes shortening from the LV lateral wall to the septum and decreases systolic stretch (mainly in the septum, see Figs. 1, 3). In Fig. 7, it can be appreciated that the gain in septal shortening is considerably larger than the decrease in LV free wall shortening. These investigators also demonstrated that of the various indices of discoordination, SRS was the most sensitive marker of the mechanical substrate for CRT response [14].
Spatial distribution of local systolic shortening amplitudes before and during CRT. Prior to CRT (gray bars), systolic shortening was highly polarized between the interventricular septum and the LV free wall. CRT homogenized distribution of systolic shortening (‘recoordination’) by strongly increasing shortening in the septal segments (Sept and AS) and slightly decreasing shortening in lateral (LAT) and posterior (Post) LV wall segments. Modified after [14]
Further improvement in pump function is possibly mediated by reduction of mitral regurgitation [10] and prolongation of diastolic filling time. These beneficial effects occur almost immediately after starting resynchronization [4], [10], but also translate to long-term effects like reverse remodeling as well as better clinical outcome, including better survival. For this article, it is important to note that reverse remodeling consists of reduction of LV dilatation as well as regression of hypertrophy in the most hypertrophied late activated LV lateral wall [53]. This reversal of dilatation and asymmetric hypertrophy appears to be related to the more uniform distribution of myocardial strains and workload in the resynchronized heart [14, 53] (Fig. 7).
Energetic consequences
Because abnormal systolic strains in dyssynchronous hearts are closely related to local energetics, it is reconfirming that upon resynchronization the distribution of blood flow, oxygen consumption and glucose uptake, as studied by PET, becomes more uniform [25, 28, 32, 33] (Fig. 8). The finding that this recovery of blood flow occurs within 2 weeks after onset of CRT [33] and disappears within 15 min after stopping CRT [25] indicates that the perfusion inhomogeneities in the LBBB hearts importantly relate to the abnormal distribution of workload (for example evidenced by the inhomogeneities in strain between septum and later wall). In addition, Knaapen et al. demonstrated that CRT also increases septal blood flow during adenosine infusion [25]. This increase in septal flow was immediately abrogated upon stopping CRT, suggesting that CRT alleviates impediment of perfusion by the abnormal contraction pattern [25]. Furthermore, an increase in hyperemic flow was found to be associated with a decrease in eccentricity of LV hypertrophy, an index of wall stress.
Myocardial blood flow during rest (MBF) and hyperemia (MBF stress, both ml/min/ml of myocardium) and the flow reserve, as measured with PET and H2O15 in patients before (baseline) and after 3 months of biventricular pacing. Data derived from table 3 of [25]
The more uniform distribution of cardiac contraction during CRT also leads to a higher efficiency of the entire LV chamber. Nelson et al. showed elegantly that resynchronization increases LVdP/dtmax while myocardial oxygen consumption even slightly decreased [31]. In these patients, a similar increase in LVdP/dtmax, evoked by dobutamine infusion, significantly increased myocardial oxygen consumption. Therefore, restoration of coordination of contraction by CRT improves cardiac function through a mechanism different from that by inotropic stimulation. The observation that CRT improves ventricular efficiency is important for two reasons. First of all, there is evidence that, in general, failing hearts have a reduced ratio between work performed and oxygen consumed (mechanical efficiency) [45]. Also, these hearts often have compromised energy metabolism, as evidenced by a reduced ratio of phosphocreatine to total ATP [15]. So, any relief of the compromised energy metabolism can be expected to have a relevant benefit.
Very few therapies in heart failure improve cardiac efficiency. While inotropic drugs increase myocardial oxygen consumption in parallel with contractility, vasodilators decrease both parameters. The effect of phosphodiesterase III inhibitors depends on the vasodilative and inotropic effects as well [20].
The improved cardiac efficiency achieved by CRT is unlikely to be due to alterations in intrinsic myocyte function. As discussed above, in canine hearts, myocardial efficiency was similarly depressed acutely and after 4 months of RV pacing [30]. Rather, most of the net effect is observed at the chamber level because of the more coordinate contraction in different regions of the LV wall. This process is analogous to that of a poorly timed automotive engine; each piston continues to burn fuel, but when timing is suboptimal, there is reduced effective engine power, wasted work and lower fuel economy [31].
Conclusions
CRT has several beneficial effects on mechano-energetics of the heart: it alleviates impediment of the abnormal contraction on blood flow and increases myocardial efficiency. These two factors act together to increase the range of cardiac work that can be delivered by the patients’ heart, an effect that can explain the increased exercise tolerance and quality of life reported in several CRT trials.
References
Amitzur G, Manor D, Pressman A, Adam D, Hammerman H, Shofti R, Beyar R, Sideman S (1995) Modulation of the arterial coronary blood flow by asynchronous activation with ventricular pacing. Pacing Clin Electrophysiol 18:697–710
Andersen HR, Nielsen JC, Thomsen PEB, Thuesen L, Mortensen PT, Vesterlund T, Pedersen AK (1997) Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet 350:1210–1216
Auricchio A, Abraham WT (2004) Cardiac resynchronization therapy: current state of the art, cost versus benefit. Circulation 109:300–307
Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, Klein H, Kramer A, Ding J, Salo R, Tockman B, Pochet T, Spinelli J (1999) Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The pacing therapies for congestive heart failure study group. The guidant congestive heart failure research group. Circulation 99:2993–3001
Badke FR, Boinay P, Covell JW (1980) Effect of ventricular pacing on regional left ventricular performance in the dog. Am J Physiol 238:H858–H867
Baller D, Wolpers H-G, Zipfel J, Bretschneider H-J, Hellige G (1988) Comparison of the effects of right atrial, right ventricular apex and atrioventricular sequential pacing on myocardial oxygen consumption and cardiac efficiency: a laboratory investigation. PACE 11:394–403
Beppu S, Matsuda H, Shishido T, Miyatake K (1997) Functional myocardial perfusion abnormality induced by left ventricular asynchronous contraction: experimental study using myocardial contrast echocardiography. J Am Coll Cardiol 29:1632–1638
Bilchick KC, Dimaano V, Wu KC, Helm RH, Weiss RG, Lima JA, Berger RD, Tomaselli GF, Bluemke DA, Halperin HR, Abraham T, Kass DA, Lardo AC (2008) Cardiac magnetic resonance assessment of dyssynchrony and myocardial scar predicts function class improvement following cardiac resynchronization therapy. JACC Cardiovasc Imaging 1:561–568
Blanc JJ, Etienne Y, Gilard M, Mansourati J, Munier S, Boschat S, Benditt J, Lurie KG (1997) Evaluation of different ventricular pacing sites in patients with severe heart failure: results of an acute hemodynamic study. Circulation 96:3273–3277
Breithardt OA, Sinha AM, Schwammenthal E, Bidaoui N, Markus KU, Franke A, Stellbrink C (2003) Acute effects of cardiac resynchronization therapy on functional mitral regurgitation in advanced systolic heart failure. J Am Coll Cardiol 41:765–770
Burkhoff D, Oikawa RY, Sagawa K (1986) Influence of pacing site on left ventricular contraction. Am J Physiol 251:H428–H435
Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L, Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators (2005) The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352:1539–1549
De Boeck BW, Kirn B, Teske AJ, Hummeling RW, Doevendans PA, Cramer MJ, Prinzen FW (2008) Three-dimensional mapping of mechanical activation patterns, contractile dyssynchrony and dyscoordination by two-dimensional strain echocardiography: rationale and design of a novel software toolbox. Cardiovasc Ultrasound 6:22
De Boeck BW, Teske AJ, Meine M, Leenders GE, Cramer MJ, Prinzen FW, Doevendans PA (2009) Septal rebound stretch reflects the functional substrate to cardiac resynchronization therapy and predicts volumetric and neurohormonal response. Eur J Heart Fail 11:863–871
De Sousa E, Veksler V, Minajeva A (1999) Subcellular creatine kinase alterations: implications in heart failure. Circ Res 85:68–76
Dekker AL, Phelps B, Dijkman B, van der Nagel T, van der Veen FH, Geskes GG, Maessen JG (2004) Epicardial left ventricular lead placement for cardiac resynchronization therapy: optimal pace site selection with pressure-volume loops. J Thorac Cardiovasc Surg 127:1641–1647
Delhaas T, Arts T, Prinzen FW, Reneman RS (1994) Regional fibre stress-fibre strain area as estimate of regional oxygen demand in the canine heart. J Physiol (London) 477(3):481–496
Delhaas T, Arts T, Prinzen FW, Reneman RS (1993) Relation between regional electrical activation time and subepicardial fiber strain in the canine left ventricle. Eur JPhysiol (Pfluegers Arch) 423:78–87
Durrer D, Dam v RT, Freud GE, Janse MJ, Meyler FL, Arzbaecher RC (1970) Total excitation of the isolated human heart. Circulation 41:899–912
Hasenfuss G, Holubarsch C, Heiss HW (1989) Myocardial energetics in patients with dilated cardiomyopathy: influence of nitroprusside and enoximone. Circulation 80:51–64
Kass DA, Chen C-H, Curry C, Talbot M, Berger R, Fetics B, Nevo E (1999) Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation 99:1567–1573
Kerckhoffs R, Omens JH, McCulloch AD, Mulligan LJ (2010) Ventricular dilation and electrical dyssynchrony synergistically increase regional mechanical non-uniformity but not Mechanical Dyssynchrony: A computational model. Circ Heart Fail 3:528–536
Kirn B, Jansen A, Bracke F, Van Gelder B, Arts T, Prinzen FW (2008) Mechanical discoordination rather than dyssynchrony predicts reverse remodeling upon cardiac resynchronization. Am J Physiol 295:H640–H646
Klimusina J, De Boeck BWL, Leenders GE, Faletra FF, Prinzen FW, Averaimo M, Pasotti E, Klersy C, Moccetti T, Auricchio A (in press) Redistribution of left ventricular strain by cardiac resynchronization therapy in heart failure patients. Eur J Heart Fail
Knaapen P, van Campen LM, de Cock CC, Gotte MJ, Visser CA, Lammertsma AA, Visser FC (2004) Effects of cardiac resynchronization therapy on myocardial perfusion reserve. Circulation 110:646–651
Leclercq C, Cazeau S, Le Breton H, Ritter P, Mabo P, Gras D, Pavin D, Lazarus A, Daubert JC (1998) Acute hemodynamic effects of biventricular DDD pacing in patients with end-stage heart failure. J Am Coll Cardiol 32:1825–1831
Leenders GE, Cramer MJ, Bogaard MD, Meine M, Doevendans PA, de Boeck BW (in press) Echocardiographic prediction of outcome after cardiac resynchronization therapy: conventional methods and recent developments. Heart Fail Rev. doi:10.1007/s10741-010-9200-8
Lindner O, Vogt J, Kammeier A, Wielepp P, Holzinger J, Baller D, Lamp B, Hansky B, Korfer R, Horstkotte D, Burchert W (2005) Effect of cardiac resynchronization therapy on global and regional oxygen consumption and myocardial blood flow in patients with non-ischaemic and ischaemic cardiomyopathy. Eur Heart J 26:70–76
Lister JW, Klotz DH, Jomain SL, Stuckey JH, Hoffman BF (1964) Effect of pacemaker site on cardiac output and ventricular activation in dogs with complete heart block. Am J Cardiol 14:494–503
Mills RW, Cornelussen RN, Mulligan LJ, Strik M, Rademakers LM, Skadsberg ND, van Hunnik A, Kuiper M, Lampert A, Delhaas T, Prinzen FW (2009) Left ventricular septal and left ventricular apical pacing chronically maintain cardiac contractile coordination, pump function and efficiency. Circ Arrhythm Electrophysiol 2:571–579
Nelson GS, Berger RD, Fetics BJ, Talbot M, Spinelli C, Hare JM, Kass DA (2000) Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation 102:3053–3059
Neri G, Zanco P, Zanon F, Buchberger R (2003) Effect of biventricular pacing on metabolism and perfusion in patients affected by dilated cardiomyopathy and left bundle branch block: evaluation by positron emission tomography. Europace 5:111–115
Nowak B, Sinha AM, Schaefer WM, Koch KC, Kaiser HJ, Hanrath P, Buell U, Stellbrink C (2003) Cardiac resynchronization therapy homogenizes myocardial glucose metabolism and perfusion in dilated cardiomyopathy and left bundle branch block. J Am Coll Cardiol 41:1523–1528
Owen CH, Esposito DJ, Davis JW, Glower DD (1998) The effects of ventricular pacing on left ventricular geometry, function, myocardial oxygen consumption and efficiency of contraction in conscious dogs. PACE 21:1417–1429
Packer M, Carver JR, Rodeheffer RJ, Group. ftPSR (1991) Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med 325:1468–1475
Peschar M, de Swart H, Michels KJ, Reneman RS, Prinzen FW (2003) Left ventricular septal and apex pacing for optimal pump function in canine hearts. J Am Coll Cardiol 41:1218–1226
Prinzen FW, Augustijn CH, Allessie MA, Arts T, Delhaas T, Reneman RS (1992) The time sequence of electrical and mechanical activation during spontaneous beating and ectopic stimulation. Eur Heart J 13:535–543
Prinzen FW, Augustijn CH, Arts T, Allessie MA, Reneman RS (1990) Redistribution of myocardial fiber strain and blood flow by asynchronous activation. Am J Physiol 259:H300–H308
Prinzen FW, Delhaas T, Arts T, Reneman RS (1998) Regional electromechanical coupling during ventricular pacing. In: Vardas PE (ed) Cardiac arrhythmias, pacing and electrophysiology. Kluwer Academic Publishers, Athens, pp 353–359
Prinzen FW, Hunter WC, Wyman BT, McVeigh ER (1999) Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol 33:1735–1742
Rodriguez LM, Timmermans C, Nabar A, Beatty G, Wellens HJ (2003) Variable patterns of septal activation in patients with left bundle branch block and heart failure. J Cardiovasc Electrophysiol 14:135–141
Russell K, Opdahl A, Remme EW, Gjesdal O, Skulstad H, Kongsgard E, Edvardsen T, Smiseth OA (2010) Evaluation of left ventricular dyssynchrony by onset of active myocardial force generation: a novel method which differentiates between electrical and mechanical etiologies. Circ Cardiovasc Imaging 3:405–414
St. John Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR, Loh E, Kocovic DZ, Fisher WG, Ellestad M, Messenger J, Kruger K, Hilpisch KE, Hill MR (2003) Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 107:1985–1990
Suga H, Hayashi T, Suehiro S, Hisano R, Shirahata M, Ninomiya I (1981) Equal oxygen consumption rates of isovolumic and ejecting contractions with equal systolic pressure-volume areas in canine left ventricle. Circ Res 49:1082–1091
Suga H, Igarashi Y, Yamada O (1985) Mechanical efficiency of the left ventricle as a function of preload, afterload, and contractility. Heart Vessels 1:3–8
Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman R, Lee KL, Lamas GA (2003) Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 107:2932–2937
Ter Keurs HEDJ, Rijnsburger WH, Van Heuningen R, Nagelsmit MJ (1980) Tension development and sarcomere length in rat cardiac trabeculae. Evidence of length-dependent activation. Circ Res 46:703–714
Tyberg JV, Parmley WW, Sonnenblick EH (1969) In vitro studies of myocardial asynchrony and regional hypoxia. Circ Res 25:569–579
Van Oosterhout MFM, Arts T, Bassingthwaighte JB, Reneman RS, Prinzen FW (2002) Relation between local myocardial growth and blood flow during chronic ventricular pacing. Cardiovasc Res 53:832–841
Vassallo JA, Cassidy DM, Marchlinski FE, Buxton AE, Waxman HL, Doherty JU, Josephson ME (1984) Endocardial activation of left bundle branch block. Circulation 69:914–923
Vassallo JA, Cassidy DM, Miller JM, Buxton AE, Marchlinski FE, Josephson ME (1986) Left ventricular endocardial activation during right ventricular pacing: effect of underlying heart disease. J Am Coll Cardiol 7:1228–1233
Verbeek XAAM, Auricchio A, Yu Y, Ding J, Pochet T, Vernooy K, Kramer A, Spinelli J, Prinzen FW (2006) Tailoring cardiac resynchronization therapy using interventricular asynchrony. Validation of a simple model. Am J Physiol 290:H968–H977
Vernooy K, Cornelussen RN, Verbeek XA, Vanagt WY, van Hunnik A, Kuiper M, Arts T, Crijns HJ, Prinzen FW (2007) Cardiac resynchronization therapy cures dyssynchronopathy in canine left bundle-branch block hearts. Eur Heart J 28:2148–2155
Vernooy K, Verbeek XA, Peschar M, Crijns HJ, Arts T, Cornelussen RN, Prinzen FW (2005) Left bundle branch block induces ventricular remodelling and functional septal hypoperfusion. Eur Heart J 26:91–98
Vernooy K, Verbeek XAAM, Peschar M, Crijns HJGM, Arts T, Cornelussen RNM, Prinzen FW (2005) Left bundle branch block induces ventricular remodeling and functional septal hypoperfusion. Eur Heart J 26:91–98
Wiggers CJ (1925) The muscular reactions of the mammalian ventricles to artificial surface stimuli. Am J Physiol 73:346–378
Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A (2002) Dual-chamber pacing or ventricular back-up pacing in patients with an implantable defibrillator. JAMA 288:3115–3123
Wyman BT, Hunter WC, Prinzen FW, Farris OP, McVeigh ER (2002) Effects of single- and biventricular pacing on temporal and spatial dynamics of ventricular contraction. Am J Physiol 282:H372–H379
Wyman BT, Hunter WC, Prinzen FW, McVeigh ER (1999) Mapping propagation of mechanical activation in the paced heart with MRI tagging. Am J Physiol 276:H881–H891
Yu CM, Chau E, Sanderson JE, Fan K, Tang MO, Fung WH, Lin H, Kong SL, Lam YM, Hill MR, Lau CP (2002) Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation 105:438–445
Zareba W, Klein H, Cygankiewicz I, Hall WJ, Goldberger JJ, Daubert JP, Gold MR, Rashtian M, Viskin S, Kargul W, Pitschner H, MiNitt S, Moss AJ (2010) CRT-D efefctiveness by QRS duration and morphology in the MADIT-CRT patients. Heart Rhythm 5S:S24
Zhang Q, Fung JW, Auricchio A, Chan JY, Kum LC, Wu LW, Yu CM (2006) Differential change in left ventricular mass and regional wall thickness after cardiac resynchronization therapy for heart failure. Eur Heart J 27:1423–1430
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10741-011-9227-5
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Prinzen, F.W., Vernooy, K., DeBoeck, B.W.L. et al. Mechano-energetics of the asynchronous and resynchronized heart. Heart Fail Rev 16, 215–224 (2011). https://doi.org/10.1007/s10741-010-9205-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-010-9205-3