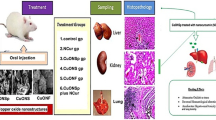
Abstract
Copper oxide Nanoparticles (CuONPs) are used in different agricultural applications. Large amounts of CuONPs cause organ dysfunction in animals. Our study aim to compare between the toxic effects of CuONanSphere (CuONSp) and CuONanoFlower (CuONF) as new nano-pesticides, determine a less toxic form when used in agricultural applications. To characterize CuONSp and CuONF, we used X-ray diffraction (XRD), Field emission scanning electron microscopy (SEM), and High resolution transmission electron microscopy (HRTEM) and Zeta-sizer device.18 adult male albino rats were divided into three groups (n = 6), (I) control group, (II) and (III) groups were given orally 50 mg/kg/day of CuONSp and CuONF 30 days respectively. CuONSp induced oxidant-antioxidant abnormalities, including an increase in malondialdhyde (MDA) and a decrease in glutathione (GSH) in comparison to CuONF-treated one. CuONSp induced an increase in liver enzymes activities compared to CuONF. Tumour necrosis factor-alfa (TNF-α) detected an increased in liver and lung compared to CuONF. However, histological examinations revealed changes in CuONSp group than CuONF group. Changes in immune-expressions of TNF-α, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kβ) and tumour suppressor gene (p53) were also more identified in CuONSp group than CuONF group. Ultrastructural studies of liver and lung tissues marked alternations were observed in CuONSp group than CuONF group. In conclusion, CuONSp induced biological alternation in liver and lung more than CuONF. So, CuONF is less toxic compared to CuONSp when used as nano-pesticide in agricultural applications.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
NPs has a lot of uses in different applications, because of their unique physicochemical characteristics such as changing their size, surface properties, and shape in application-specific ways (Aillon et al. 2009). CuO NPs have been proposed for use in soils as fertilisers, fungicides, and pesticides in order to increase agricultural sustainability (Pelegrino et al. 2020). CuO nanostructures with a variety of morphologies have been synthesised, including nanospheres, nanorods, nanoneedles, nanoflowers and nanotips. The CuO nanostructures were synthesised using a variety of methods, ranging from microwave to hydrothermal synthesis (Zhou et al. 2006). Nonetheless, their unique physicochemical characteristics can enhance their toxicological effect in vivo by promoting particle cell uptake and translocation through blood circulation (Kang et al. 2013). In vitro investigations on human cells exposed to CuO NPs revealed high cytotoxicity as well as the ability to produce DNA damage, oxidative stress, and cell death (He et al. 2020).
The physiological environment has a significant effect on NPs metabolic pathways, which will affect their existence and biosecurity (Yallapu et al. 2015). The extensive usage of NPs has raised concerns about the health dangers and environmental problems that come with their use (Johnson et al. 2015). Furthermore, the absence of biosafety assessments limited the use of NPs (Oberdörster et al. 2005).
The decrease in blood antioxidant activities caused by CuO NP exposure reveals the severity of the oxidative stress detected (Tulinska et al. 2022). The toxicity of NPs and the excessive growth of reactive oxygen species (ROS) have been related to oxidative stress, and caused apoptosis could be one of the NPs possible toxicity pathways (Benameur et al. 2015). The activities of both ALT and AST increased in blood serum. The release of liver enzymes into the blood serum is considered as an indication of liver disease or histological toxic damage (Miron and Mahbubeh 2014). The glutathione peroxidase (GPX) activity decreased in cells exposed to CuONPs. CuONPs decrease GPX activity, indicating that they not only create free radicals but also effect on the antioxidant cell resistance (Fahmy and Cormier 2009). Overproduction of ROS disrupts the balance of the liver’s oxidative and antioxidant processes, resulting in an increased in lipid peroxidation by MDA and caused hepatocyte death (Brown et al. 2004). The serum level of TNF-α was increased in CuONPs treated rats (Abdelazeim et al. 2020).
The semipermeable, discontinuous endothelium of the hepatic sinusoids and blood arteries, which allows circulating NPs of less than 100 nm to enter the hepatic parenchyma by opsonisation, may be linked to the extensive bioaccumulation of CuONPs in the liver (Sadauskas et al. 2007). The cytotoxicity of liver caused by CuONPs may involve autophagy or cell egodigestion, which is a conserved mechanism involved in the breakdown of proteins and organelles by the cytoplasm (Baehrecke 2005). CuO NPs toxicity has been linked to oxidative stress and CuO propensity to destroy mitochondria in liver cells (Karlsson et al. 2009). CuONPs are similarly exceedingly toxic, also, causing severe pulmonary edoema and mortality (Yokohira et al. 2009). CuONPs also induced apoptosis in adenocarcinomic lung epithelial cell line in lung tissues in a dose-dependent manner (Xiaofeng et al. 2018). CuONPs revealed alterations in the lung, such as edematous expansion of membranous pneumocytes and endothelial cells in some areas (Dumkova et al. 2016). Cells are known to reverse the overwhelming oxidative stress response through increased expression of cytokines such as TNF-α, kinase activation, and phosphatase inhibition, thus affecting the cascade of phosphorylation (Genestra 2007). Several metal oxide NPs increase the activation of NF-kβ pathways (Smith et al. 2001). Expressions of the p53 protein level up-regulated on exposure to CuONPs (Gopinath et al. 2010).
In the previous studies, liver and lung toxicity was induced by CuO-Nanosphere, but CuONF not used in biological applications. So, the present study aims to compare between the toxicological effects of CuONSp and CuONF as a new nano-pesticides, since no studies performed on the CuONF form to know the less toxic form which can be applied in agriculture field.
Materials and methods
Method of CuONanSphere (CuONSp) synthesis
We used the hydrothermal method in synthesis of CuONSp. Cu (NO3)2.3H2O and NaOH were dissolved in pure water and then transferred to an autoclave under steady stirring. The autoclave was kept at 170 ºC for 24 h. The autoclaves were brought to room temperature by being cooled in the air. The recovered precipitates are centrifuged, washed several times with pure water and ethanol, and then dried for 24 h in a drying oven at 60 ºC (Wang et al. 2014).
Method of CuONanoFlower (CuONF) synthesis
We used the hydrothermal method in synthesis of CuONF. In deionized water, CuCl2 was dissolved. The CuCl2 solution was then gently added to the NaOH solution, while stirring vigorously, yielding a blue-colored precursor. The blue precursor has been introduced to cetyl trimethyl ammonium bromide (CTAB) and forcefully stirred to ensure complete dissolution of CTAB. This reaction solution was then transferred to autoclave with and heated in an electric oven120 °C for 6 h. After the reaction, the autoclave was allowed to cool to room temperature. The dark precipitate was centrifuged and washed thoroughly with deionized water and ethanol. The precipitate was then dried in a drying oven for 24 h in a drying oven at 60 °C (Zou et al. 2011).
Characterizations of CuONSp and CuONF
1-X-ray diffraction (XRD) measurements of CuONSp and CuONF
X-ray diffraction (XRD) patterns were used to investigate the crystalline structure of CuONSp and CuONF. At room temperature, the XRD pattern of two shapes of CuO NPs was obtained using a PAN alytical X’Pert X-ray diffractometer fitted with a Ni filtered Cu Kα (λ = 1.54056 ºA) radiations as the X-ray source. The measurements were carried out with 2θ range 10 < θ < 80 with step size 0.04.
2-SEM and HRTEM measurements
In this study, samples were measured by SEM (JSM5610LA/ Japan) and HRTEM (JEM2100/ Japan) at Beni-Suef University to study the morphology and size of CuONSp and CuONF.
3-Nano-measurements by the Zeta-sizer device
The average hydrodynamic size, PDI and zeta potential of CuONSp and CuONF in double distilled water were estimated using dynamic light scattering (DLS) (Nano-Zeta-Sizer-HT, Malvern Instruments, Malvern, UK) at room temperature (Murdock et al. 2008).
Experimental animals
Eighteen Wistar male albino rats weighing 120–150 g were used in this study. They were purchased from the Egyptian Organization for Biological Vaccine Production (A.R.E.). They were housed in stainless steel cages at room temperature (25 °C) and on a natural light/dark cycle, with complete nutrition pellets and water available at all times. Prior to the start of the experiment, all of the animals were isolated for ten days. All animals were isolated for 10 days prior to the start of experiment. The study was approved by the Ethics Committee of the Beni-Suef University (BSU-IACUC, No. 019–78).
Animals grouping
Animals were assigned into three groups (n = 6); the first group (G1) was the untreated control group, the second (G2) and third groups (G3) were received 50 mg/kg/day of CuONSp and CuONF respectively for 30 days according to Arafaa et al. (2017). At the end of experiment the animals were sacrificed.
Biochemical studies
Blood sampling
At the end of the treatment, blood samples were taken from rats from various groups and blood samples were taken in tubes (Halperim et al.1951).
Liver functions
The activities of AST, ALT, and GGT were measured at the end of the experiment method in serum (Schumann and Klauke 2003). The DxC uses an enzymatic rate technique to determine GGT activity in serum. The rate of change in absorbance is proportional to the amount of GGT activity in the sample (Beckman et al. 2007).
Oxidative stress and TNF-α measurements
Lipid peroxidation is measured as MDA. The amount of MDA in the liver and lung was measured according to Ohkawa et al. (1979) procedure. The activity of GPX in hepatic and pulmonary was investigated according to Beutler et al. (1963) process. Rat TNF-ELISA Kit (CSB-E11987r) was used to measure TNF-α activity in the liver and lung.
Histological preparations
At the end of treatment, animals from each group were anaesthetized with light diethyl ether and dissected to remove the liver and lung for histological preparations. Parts 4 to 5 μm thick were made using a microtome and stained with haematoxylin and eosin for histological examinations, according to Bancroft and Gamble (2002).
Immunohistochemical and morphometric study
Other sections of liver and lung were mounted on + ve charged slides for immunohistochemical inspection. The sections put in 3% H2O2 followed by citrate buffer. The sections were probed with an antibody against TNF-α, NF-kβ and p53 then washed in phosphate buffer and incubated with the secondary antibody. The sections were counter-stained with Mayer,s haematoxylin. We used Leica Qwin 500 LTD image analysis (Cambridge UK) in our morphometric measurements. The area % of TNF-α, NF-kβ and p53 immunoreactivity was recorded in immuno-stained sections. The measurements were done in 10 random high powers (X400) nono-overlapping fields for each section using the binary mode.
Ultrastructural preparations
Slices of liver and lung tissue were fixed in 3% glutaraldhyde- formaldhyde, pot-fixed in osmium-tetroxide according to Bozzola and Russell (1999). The ultra-microtome glass knives were then used to cut ultrathin sections, which were dyed with uranyl acetate and lead citrate (Reynolds 1963) then was viewed at an accelerating voltage with a Joel CX 100 transmission electron microscope (TEM).
Statistical analysis
The Statistical Package (SPSS for WINDOWS, version 20.0; SPSS Inc, Chicago) was used in the social sciences (IBM Corp, 2011). Results were expressed as mean ± standard error and values of P > 0.05 were considered non-significantly different, while those P < 0.05 and P < 0.01 were significant and high significant differentiation, respectively.
Results
Our study illustrates a comparison study between the toxicological effects of CuONSp and CuONF on the liver and lung of male albino rats.
X-ray diffraction (XRD) measurements of CuONSp and CuONF
The diffraction patterns of the prepared nanostructures are illustrated in (Fig. 1a, b). The XRD patterns of (Fig. 1a) were compared and indexed with the standard JCPDS file number 96-901-5925.The data revealed the formation of CuO nano structures in monoclinic symmetry with Space group C12/c1 (Table S1). In Fig. 1b the XRD patterns were compared and indexed with the standard JCPDS file number 96-901-5823. Also, the data revealed the formation of CuO nano structures in monoclinic symmetry with Space group C12/c1 (Table S2). No extra peaks were observed pointing to the single plane structure.
The crystallite size of the samples under investigation was computed using Debye schetter formula (Ahmed et al. 2016) L\(=\frac{k\lambda }{\beta COS \theta }\) ; where k is the shape factor, λ is the target wave length; β is the corrected full width of half maximum and θ is the diffraction angle. The CuONSp was found to be 20.6 nm. While the CuONF was117.1 nm.
SEM and HRTEM measurements
To describe morphological and microstructural features to CuONSp and CuONF. We used SEM to reveal the presence of agglomerated spherical grains in CuONSp (Fig. 2a) and agglomerated grains in CuONF (Fig. 2b). Modifying the shape of the surface reduced the amount of interaction between NPs and cells. The more the shape surface of NPs is modified, the less contact with cells. As a result, we expected CuONSp has higher toxicity than CuONF.
From HRTEM the size of CuONSp was observed range of 9 nm (Fig. 3a), while, CuONF was measured at range 228 nm (Fig. 3b). One of the key factors contributing to the toxic impact of NPs was their size. The smaller in nanosize, the higher in toxicity, and as seen in the previous data, CuONSp nanosize is smaller than CuONF. Therefore, CuONSp is expected to higher toxicity than CuONF.
Nano-measurements by the Zeta-sizer device
The hydrodynamic size of two different shapes in doubled distilled water was measured in CuONSp at range 284.7 ± 59.7 d.nm, (Fig. 4a) and in CuONF at range 363 ± 82.6 d.nm (Fig. 4b). As a result, we expected the toxicity of CuONSp to be higher than CuONF, due to its small size.
The polydispersity index (PDI) was used to determine NPs stabilityin suspension.The PDI in doubled distilled water was found in CuONSp at 0.43 and in CuONF at 0.64, indicating a stable suspension. The PDI illustrates NPs size distribution, a lower value indicates more particle stability and more toxicity. Therefore, it appears that the CuONSp is more widely distributed and harmful than CuONF.
Zeta Potential of CuONSp in doubled distilled water measured at − 50.9 ± 6.5 mV (Fig. 5a) and in CuONF at range − 47.2 ± 7.6 mV (Fig. 5b). As a result, CuONSp has more charges than CuONF. The interactions of NPs with biological systems are determined by their surface charge. More toxicity was caused by a significant amount of surface charge. As a result, we believed CuONSp is more toxic than CuONF.
Biochemical effects of CuONSp and CuONF on liver functions, oxidative stress and TNF-α
CuONSp induced increase in serum AST activity with high significant (P < 0.01) (194.6 ± 6.3U/l), and CuONF at 144.2 ± 5.5 U/l compared to normal control ones (142.1 ± 6.2 U/l) at the end of experiment (Fig. 6). Also, CuONSp induced an elevation in serum ALT activity with high significant (P < 0.01) (101.8 ± 5.2U/l) and CuONF measured at 63.2 ± 6.6U/l compared to normal control ones (61.7 ± 3.1U/l) at the end of experiment. In addition, CuONSp induced arise in serum GGT activity with high significant (P < 0.01) (3.4 ± 0.18U/l) and CuONF at 2.4 ± 0.1 U/l compared to normal control rats (2.29 ± 0.1 U/l) at the end of experiment. As above result, the elevation of AST, ALT & GGT activities in CuONSp more than CuONF compared to control group (Fig. 6). This mean CuONSp induced more toxicity on liver function more than CuONF.
The oxidative stress measured as lipid peroxidation by MDA, CuONSp induced an increase in serum MDA level with high significant (P < 0.01) (97.52 ± 8.4 nmol/100 mg) and CuONF at 54.7 ± 4.6 nmol/100 mg compared to normal control (42.07 ± 4.4 nmol/100 mg). The elevation of CuONSp more than CuONF compared to control one. While in the measurements of antioxidant enzymes such as GSH, CuONSp induced a decrease in serum GSH activity with high significant (P < 0.01) (22.89 ± 1.3 U/g) and CuONF at 48.1 ± 1.4 U/g compared to normal control ones (55.34 ± 1.5 U/g). The inhibition to GSH activity by CuONSp is more than by CuONF compared to control (Fig. 7).
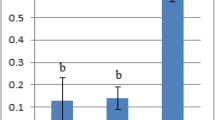
CuONSp induced increase in TNF-α level with high significant (P < 0.01) (88.57 ± 4.6 Pg/ml) and CuONF at (33.97 ± 3.1 Pg/ml) compared to normal control ones (23.23 ± 1.9 Pg/ml) at the end of experiment. As result, CuONSp induced an elevation in TNF-α level more than CuONF compared to control ones (Fig. 8).
Histopathological study of liver and lung
Microscopic examination of liver and lung control rats showed normal structure of both liver and lung (Fig. 9a, b) respectively. At the end of experiment, CuONSp rats sections showed proliferation of bile ductules, newly formed of bile ductules surounded with mononuclear leukocytic infiltration (Fig. 9c). In lung rats sections treated with CuONSp showed thickened pulmonary blood vessel, marked hyperplasia of dilated bronchiole, pyknotic nuclei, and focal areas of collapsed alveoli and thickened interalveolar septum (Fig. 9d). In liver rats sections treated with CuONF showed the structure of liver near to normal (Fig. 9e) and in lung rats sections treated with CuONF appeared to be normal (Fig. 9f).
Photomicrographs of liver and lung of rats showing a normal structure of liver rats revealing; central vein (CV), sinusoids (S) and hepatocytes (H). b normal structure of lung rats showing; normal bronchiole (arrow), blood vessel (B), alveolar sac (s), alveoli (A), interalveolar septum (arrow head). liver rats sections treated with CuONSp showing c proliferation of bile ductules (BD), newly formed of bile ductules surounded with mononuclear leukocytic infiltration (arrows), and appearance some of pyknotic nuclei. d lung rats sections treated with CuONSp showing d thickened pulmonary blood vessel (B), marked hyperplasia of dilated bronchiole (arrow head), pyknotic nuclei (arrow), and focal areas of collapsed alveoli (Ca) and thickened interalveolar septum (wave arrow). While liver rats sections treated with CuONF. e showed improvements in structure of liver including the central vein (CV), hepatocytes (H) and sinusoids (S). Also, lung rats sections treated with CuONF. f illustrating improvement of blood vessel (arrow head), bronchiole (arrow) and alveoli (A). (H & E; Scale bar = 50 μm)
Immunohistochemical and morphometric analysis of liver and lung
Immuno-histochemical expression of control liver demonstrated negative TNF-α (Fig. 10a), NF-kβ (Fig. 11a) and P53 (Fig. 12a) immune-expression. CuONSp treated group demonstrated strong positive immune-reactivity of TNF-α (Fig. 10c), NF-kβ (Fig. 11c) and P53 (Fig. 12c). CuONF-treated group revealed mild positive immune-reactivity of TNF-α (Fig. 10e), NF-kβ (Fig. 11e) and P53 (Fig. 12e). Liver of CuONSp-treated group induced a significant increase in mean area % of TNF-α (Fig. 10g), NF-kβ (Fig. 11g) and P53 (Fig. 12g) than CuONF-treated group compared to normal control ones.
Photomicrographs of immunohistochemical expression of in liver and lung sections in CuONSp & CuONF treated groups showing a, b Negative immunohistochemical expression of TNF-α in control group in liver &lung respectively. c, d Strong positive immune-reactivity in cytoplasm of TNF-α in CuONSp treated group in liver and lung respectively. e, f Mild immno-expression for TNF-α of CuONF treated group in liver &lung respectively. (TNF-α; Scale bar = 50 μm). Bar charts showing the mean area of TNF-α immunostaining in liver and lung sections of CuONSp & CuONF respectively. a,b,c indicated the difference or similarity between groups. Group with different superscript letters are considered significantly different (P < 0.05). Groups with the same superscript letter did not present any significant differences
Photomicrographs of immunohistochemical expression of in liver and lung sections in CuONSp & CuONF treated groups showing a, b Negative immunohistochemical expression of NF-kβ in control group in liver &lung respectively. c, d Strong positive immune-reactivity in nuclei of NF-kβ in CuONSp treated group in liver and lung respectively. e, f few immno-expression for NF-kβ of CuONF treated group in liver &lung respectively. (NF-kβ; Scale bar = 50 μm). g, h Bar charts showing the mean area of NF-kβ immunostaining in liver and lung sections of CuONSp & CuONF respectively. a,b,c indicated the difference or similarity between groups. Group with different superscript letters are considered significantly different (P < 0.05). Groups with the same superscript letter did not present any significant differences
Photomicrographs of immunohistochemical expression of in liver and lung sections in CuONSp & CuONF treated groups showing a, b Negative immunohistochemical expression of P53 in control group in liver &lung respectively. c, d Strong positive immune-reactivity in nuclei of P53 in CuONSp treated group in liver and lung respectively. e, f few immno-expression for P53 of CuONF treated group in liver &lung respectively. (P53; Scale bar = 50 μm). g, h Bar charts showing the mean area of P53 immunostaining in liver and lung sections of CuONSp & CuONF respectively. a,b,c indicated the difference or similarity between groups. Group with different superscript letters are considered significantly different (P < 0.05). Groups with the same superscript letter did not present any significant differences
Lung of control group showed negative TNF-α (Fig. 10b), NF-kβ (Fig. 11b) and P53 (Fig. 12b) immune-expression. CuONSp treated group of pulmonary tissue revealed intensive positive immune-reactivity of TNF-α (Fig. 10d), NF-kβ (Fig. 11d) and P53 (Fig. 12d). Lung CuONF-treated group illustrated few positive immune-reactivity of TNF-α (Fig. 10f), NF-kβ (Fig. 11f) and P53 (Fig. 12f). Lung CuONSp treated group induced an elevation in mean area % of TNF-α (Fig. 10h), NF-kβ (Fig. 11h) and P53 (Fig. 12h) with marked significant than CuONF compared to the normal control ones.
Ultrastructural examination of liver and lung after exposure to CuONSp and CuONF
Control liver demonstrated a normal structure (Fig. 13a) and lung control group revealed normal structure (Fig. 13b). Hepatocytes treated with CuONSp revealed nucleus with irregular nuclear envelope, numerous swollen mitochondria with ill-defined cristae and variable-sized fat droplets (Fig. 13c). Lung treated with CuONSp showed irregular pyknotic nucleus of pneumocyte type 2 with empty lamellar bodies, degenerated mitochondria lack of microvilli and deposition of collagen fibres (Fig. 13d). Hepatocytes treated with CuONF, revealed less toxicity compared to CuONSp group, the nucleus appeared with normal chromatin pattern, nucleolus and nuclear envelope, Mitochondria also appeared with well-defined cristae (Fig. 13e). Lung treated with CuONF showed nearly normal nucleus, mitochondria and intact microvilli on the surface (Fig. 13f).
An Electron micrograph of rat liver; a Control group demonstrating a normal hepatocyte with rounded euchromatic nucleus (N), prominent nuclear membrane (arrow), regular rough endoplasmic reticulum (arrow head), and many round or oval mitochondria (M) with normal cristae. (TEM, Scale bar = 2 μm). b Electron micrographs of ultrathin section of control rat lung tissue shows nucleus (N) of pneumocyte type 2, lamellar bodies (L), around the nucleus, mitochondria (M), microvilli (mv) on the surface. (TEM, Scale bar = 2 μm). c Showing hepatocytes after treatment with CuONSp for 30 days, Nucleus (N) with irregular nuclear envelope (arrow), numerous swollen mitochondria (M) with ill-defined criste, variable-sized fat droplets (arrow head) (TEM, Scale bar = 2 μm). d Electron micrographs of ultrathin section of CuONSp treated group rat lung shows irregular pyknotic nucleus (N) of pneumocyte type 2 with empty lamellar bodies (L), degenerated mitochondria (M) lack of microvilli (mv). Notice the deposition of collagen fibres (C) (TEM, Scale bar = 2 μm). e hepatocytes treated with CuONF, revealing less toxicity compared to CuONSp group, the nucleus appear with normal chromatin pattern, nucleolus (N) and nuclear envelope (arrow), and mitochondria well-defined cristae (M) (TEM, Scale bar = 2 μm). f Lung treated with CuONF shows nearly normal nucleus (N), mitochondria (M) and intact microvilli (mv) on the surface (TEM, Scale bar = 2 μm)
Discussion
Engineered NPs that have gained the most attention in other sectors have very little potential for large-scale agricultural applications (e.g., carbon nanotubes, nanosilver) (Aschberger et al. 2015) NPs are commonly used in nano-technology with a size < 100 nm (Linic et al. 2015). In our study, we used hydrothermal methods to produce two different shapes of CuONPs, including CuONSp and CuONF. Through synthesis of CuONSp, Cu (OH)2 precipitate is then converted in the nano-sphere under hydrothermal conditions; this result is in agreement with (Seo et al. 2013). Furthermore in preparation of CuONF, we used CTAB acts as a stabilizer for the synthesis of CuONF through hydrothermal process (Zhou et al. 2006).
In the XRD measurement of our study, the crystallite size was detected for CuONSp at 20.6 nm and recorded at 117.1 nm in CuONF. The absence of any other peaks indicates that the samples are pure. The increased sharpness of XRD peaks and the grain size observed for CuONPs using the relative intensity peak suggest that the particles are crystalline in nature. According to Mohammadyari et al. (2014) the grain size of CuONPs determined by the relative intensity peak was 50 nm, and an improvement in XRD peak sharpness indicates that the particles are in crystalline form. According to our findings, the alteration of NPs shape, which reduces the degree of interaction between cells and NPs. The lower contact with the cells, the most modified shape surface of the NPs changes, which explain that toxicity induced by CuONSp is more than by CuONF. Vasilakes et al. (2013) indicates that the shape of NPs is just as essential as their size in the passage of a drug through the body.
Our HRSEM measurements revealed the presence of aggromerated particles in two different shapes of CuONPs. Although the size of CuONSp was observed by HRTEM at rang 9 nm, it is under the category of quantum dots (QDs). CuONF has a mean range of 228 nm. As a result, CuONSp has smaller QD size in comparison to CuONF, CuONSp is the most toxic from CuONF. Oxidative stress is caused by the entry of QDs particles into cells and the production of free NPs ions (Ambrosone et al. 2012). There is proof that QDs penetration creases the fluidity of the cell membrane (Wang et al. 2012).
Our measurements of PDI to CuONSp detected at 0.43 and CuONF at 0.64. That CuONSp have the greatest particle stability and distibution from CuONF. These findings, confirmed by Masarudin et al. (2015) who stated that the PDI was used as an indicator of the stability and uniformity of nanoparticle formation. While, the lowest value in PDI the most stable in suspension. Our Zeta Potential measurements for CuONSp charge measured at − 50.9 ± 6.5mV and CuONF at − 47.2 ± 7.6mV. The surface charge of NPs plays an important role in their toxicity, since the interactions of NPs with biological systems are largely determined. More toxicity was caused by a significant amount of surface charge. As a result, the toxicity of CuONSp is the most toxic relative to CuONF. Sharifi et al. (2012) revealed that, the cationic surface charge, once internalized, functions as a proton sponge that inhibits normal lysosomal activity and initiates cell death.
It has been hypothesized that CuO nano-sized catalytic properties may be linked to part of the ROS generation. Over production of ROS will disturb the balance of the oxidative / antioxidant mechanism of the liver, resulting in lipid peroxidation through the creation of ROS and MDA and hepatocyte apoptosis, which may be closely linked to the reduction of antioxidant enzymes (Brown et al. 2001). The decrease in cell viability observed could be due to an increase in oxidative stress after exposure to CuONPs (Abudayyak et al. 2020). In the current study, CuONPs were orally treated to rats at a dose 50 nm at two different shapes; CuONSp induced an elevation MDA level more than CuONF compared to control. Also, CuONSp induced GSH inhibition activity of more than CuONF in compared to control. The toxicity of metal NPs and the excessive growth of ROS, oxidative stress have been involved and induced apoptosis may be one of the possible toxicity mechanisms of NPs (Benameur et al. 2015). Also, Rossner et al. (2020) said that employed next generation sequencing protocols and determined gene expression changes in the lungs of mice exposed to inhalation of CuO NPs. In rats intoxicated with CuONPs, hepatic levels of MDA were significantly elevated compared to normal rats and a decrease in activity of GSH in the liver relative to normal rats (Abdelazeim et al. 2020; Boyadzhiev et al. 2021) reported that the up-regulation of DNA was presented in a recent transcriptomics, where mouse lung epithelial cells were exposed to varying doses of CuO NPs for different periods of time.
Our biochemical analysis of CuONSp and CuONF groups revealed that CuONSp induced an elevation in AST, ALT &GGT activities with a high significant than CuONF. These in accordance with Rabia et al. (2019) who suggested that CuONPs displayed histological changes which correlated with the recorded increased in liver AST and ALT. According to Pietrofesa et al. (2021) inhalation of CuO NPs causes an inflammatory reaction that damages cells and lung tissues. In the current microscopic observations, the liver of CuONSp treated rats showed proliferation of bile ductules, newly developed bile ductules surrounded by mononuclear leukocytic infiltration at the end of experiment. However, in CuONF treated rats, liver nearly appeared with normal structure. In CuONSp treated lung rats, thickened pulmonary blood vessel, pronounced hyperplasia of dilated bronchiole, pyknotic nuclei, and focal areas of collapsed alveoli and thickened interalveolar septum. However, CuONF treated rats; lung appeared near to normal structure. The amount of Cu in lungs and liver was significantly increased after six weeks of CuO NPs exposure (Tulinska et al. 2022; Dumková et al. 2017) found that major changes in the liver occurred during sub chronic exposure to lead oxide nanoparticles (PbO NPs), primarily manifested by hepatocyte enlargement and hydropic degeneration. Also, inhalation of PbO NPs induced significant alterations in lung morphology following six weeks exposure. Lungs of these animals displayed mild hyperaemia, congested capillaries, swollen pulmonary septa, haemostasis in basal parts of lobes with involvement of several siderophages (macrophages with pigment hemosiderin) and the presence of alveolar emphysema in certain parts of the lungs. Valentini et al. (2019) reported that Titanium oxide nanoparticles (TiO2-NPs) have entered the liver via blood circulation following intraperitoneal injection. Kupffer cells, possibly phagolysosomes, located in hepatic sinusoids as well as in the periphery of the portal tract, were internalized with NP aggregates. These aggregates emerged as spherical inclusions of the refringent. NPs were also present in some hepatocytes in dense cytoplasmic inclusions.
Our immunohistochemical staining of TNF-α, NF-kβ & P53 in liver and lung sections revealed, strong positive expression of TNF-α, NF-kβ & P53 immune-expressions in CuONSp treated rats, but mild expression was observed in liver and lung sections CuONF-treated rats. Moreover, in the present examination, CuONSp caused a rise in serum TNF-α level more than with CuONF (Abdelazeim et al. 2020). The inflammatory cascade includes profibrotic mediators that have been implicated in fibrosis pathogenesis, such as TNF-α. Cells are known to reverse the overwhelming oxidative stress response through increased expression of cytokines such as TNF-α, kinase activation, and phosphatase inhibition, thus affecting the cascade of phosphorylation (Genestra 2007). Several metal oxide NPs increase the activation of NF-kβ pathways (Smith et al. 2001). During stressful conditions, such as ROS-mediated DNA damage, p53 can protect the genome. In order to allow time for damage to be repaired, the primary role of the genome is to cause cell cycle arrest. The cell responds by switching to apoptosis if the DNA damage cannot be repaired (Li et al. 2012).
In our ultrastructural studies, hepatocytes treated with CuONSp revealed nucleus with irregular nuclear envelope, numerous swollen mitochondria with ill-defined cristae, variable-sized fat droplets. Lung treated with CuONSp showed irregular pyknotic nucleus of pneumocyte type 2 with empty lamellar bodies, degenerated mitochondria lack of microvilli and deposition of collagen fibres. While, hepatocytes treated with CuONF, revealed less toxicity compared to CuONSp- treated group, the nucleus appear with normal chromatin pattern, nucleolus and nuclear envelope. Also, appear with mitochondria well-defined cristae. Also, lung treated with CuONF showed nucleus near to normal pneumocyte type 2, mitochondria in cytoplasm with microvilli on the surface. Semisch et al. (2014) demonstrated that the damage of mitochondrial membrane of liver cells can be generated by direct interactions with CuONPs or by ROS release, leading to apoptotic enzyme discharge. ROS generation induced by NPs causes harmful to DNA, proteins and organelles, including mitochondria. Damaged mitochondria cause intrinsic and subsequently extrinsic apoptotic pathways to be activated (Ou et al. 2016; Roda et al. 2019) revealed that the early initiation of inflammation has been detected, as evidenced by the increased presence of various inflammatory cells, including multiple activated Kupffer cells and phagosomes. Furthermore, a fibrotic reaction has been demonstrated by an evident deposition of collagen fibres. Gaharwar et al. (2019) suggested that exposure to NPs induced vacuolated mitochondria and lung tissue damage in compared to the control tissue. The surfactant in the alveoli encased by NPs enhanced tubular myelin production was associated with increased surfactant production by alveolar epithelial type 2 cells (PII).
Conclusion
CuONSp and CuONF were synthesised using hydrothermal methods in our research. CuONSp and CuONF were found to be crystalline in XRD measurements. The smaller the particles, the higher their toxicity. CuONSp has smaller particles than CuONF, so it is more toxic than CuONF. The PDI was used to determine the stability of NPs, a lower value indicates more stability and more toxicity. The value of PDI for CuONSp is lower than for CuONF. So, it is more toxic than CuONF. Also, the toxicity was determined by the amount of surface charges on nanoparticles. CuONSp has more charges than CuONF, so it has more toxicity. Around our biochemical alternations induced by CuONPs. There were an elevation in MDA level, AST, ALT & GGT activities. The Bax level is more in CuONSp than CuONF. As well as, an inhibition in GSH, SOD and CAT activities as well as in Bcl2 level induced by CuONSp < CuONF. Histopathological and ultrastructural studies in liver & lung tissues induced marked alternations by CuONSp > CuONF. Our immune-expression of TNF-α, NF-Kβ & P53 in liver and lung sections in various treatment groups, showing intense positive immune-expression of TNF-α, NF-Kβ & P53 expressions in the CuONSp treated group, mild positive TNF-α, NF-Kβ & P53 expressions in the CuONF treated group. Therefore, according to the present results CuONSp & CuONF and the toxicity induced in different studies, CuONF is better in use than CuONSp in agriculture applications due to its lower toxicity. The present study suggests that further studies are required to investigate its safety and toxicity compared to other form.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdelazeim SA, Shehata NI, Aly HF et al (2020) Amelioration of oxidative stressmediated apoptosis in copper oxide nanoparticlesinduced liver injury in rats by potent antioxidants. Sci Rep 10:10812. https://doi.org/10.1038/s41598-020-67784-y
Abudayyak M, Guzel E, Özhan G (2020) Cupric oxide nanoparticles induce cellular toxicity in liver and intestine cell lines. Adv Pharm Bull 10(2):213–222. https://doi.org/10.34172/apb.2020.025
Ahmed HH, Mansour SF, El-Dek SI et al (2016) Hybridization between microstructure and magnetization improvement lead and RECO doped Bi Feo3. J Rare Earths 34(5):195–506
Allion KL, Xie Y, El-Gendy N et al (2009) Effects of nanomaterial physicochemical properties in vivo toxicity. Adv Drug Deliv Rev 61(6):457–466. https://doi.org/10.1016/j.addr.2009.03.010
Ambrosone A, Mattera L, Marchesano V et al (2012) Mechanisms underlying toxicity induced by Cd Te quantum dots determined in an invertebrate model organism. Biomaterials 33(7):1991–2000. https://doi.org/10.1016/j.biomaterials.2011.11.041
Arafaa AF, Ghanema HZ, Solimana MS et al (2017) Modulation effects of quercetin against copper oxide nanoparticles-induced liver toxicity in rats. Egypt Pharma J 16:78–86. https://doi.org/10.4103/epj.epj_15_17
Aschberger K, Gottardo S, Amenta V et al (2015) Nanomaterials in food current and future applications and regulatory aspects. J Phys Conf Ser 617:1–6. https://doi.org/10.1088/1742-6596/617/1/012032
Baehrecke EH (2005) Autophagy: dual roles in life and death? Nat. Rev Mol Cell Biol 6:505–510. https://doi.org/10.1038/cdd.2008.120
Bancroft JD, Gamble M (2002) Theory and practice of histological techniques, 5th edn. Library Catalogue, New York
Beckman RA, Weiner LM, Davis HM (2007) Antibody constructs in cancer therapy. ASC J 109(2):170–179. https://doi.org/10.1002/cncr.22402
Benameur L, Auffan M, Cassien M et al (2015) DNA damage and oxidative stress induced by CeO2 nanoparticles in human dermal fibroblasts: evidence of a clastogenic effect as a mechanism of genotoxicity. Nanotoxicol 9:696–705. https://doi.org/10.3109/17435390.2014.968889
Beutler E, Duron O, Mkelly B (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888 PMID: 13967893
Boyadzhiev A, Avramescu ML, Wu D et al (2021) Impact of copper oxide particle dissolution on lung epithelial cell toxicity: response characterization using global transcriptional analysis. Nanotoxicology 28:1–20. https://doi.org/10.1080/17435390.2021.1872114
Bozzola JJ, Russell LD (1999) Electron microscopy: principles and techniques for biologists. Jones & Bartlett Learning, Sudbury, MA. http://people.uncw.edu/Borretts/courses/hon120/Electron%20microscopy.pdf
Brown DM, Wilson MR, MacNee W et al (2001) Size-dependent pro-inflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol pharmacol 175(3):191–199. https://doi.org/10.1006/taap.2001.9240
Brown DM, Donaldson K, Borm PJ et al (2004) Calcium and ROS—mediated activation of transcription factors and TNF-α cytokine gene expression in macrophages exposed to ultrafine particles. Am J Physiol Lung Cell Mol Physiol 286:344–353. https://doi.org/10.1152/ajplung.00139.2003
Dumková J, Smutná T, Vrlíková L et al (2017) Sub-chronic inhalation of lead oxide nanoparticles revealed their broad distribution and tissue-specific subcellular localization in target organs. Part Fibre Toxicol 14:55. https://doi.org/10.1186/s12989-017-0236-y
Dumkova J, Vrlikova L, Vecera Z et al (2016) Inhaled cadmium oxide nanoparticles: their in vivo fate and effect on target organs. Int J Mol Sci 17(6):874. https://doi.org/10.3390/ijms17060874
Fahmy B, Cormier SA (2009) Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol 23:1365–1371. https://doi.org/10.1016/j.tiv.2009.08.005
Gaharwar US, Meena R, Rajamani P (2019) Biodistribution, clearance and morphological alterations of intravenously administered iron oxide nanoparticles in male Wistar rats. Int J Nanomed 14:9677–9692. https://doi.org/10.2147/IJN.S223142
Genestra M (2007) Oxylradicals,redox-sensitivesignallingcascades and antioxidants. Cell Signal 19(9):1807–1819. https://doi.org/10.1016/j.cellsig.2007.04.009
Gopinath P, Gogoi SK, Sanpui P (2010) Signaling gene cascade in silver nanoparticle induced apoptosis. Colloids Surf Biointerfaces 77(2):240–245. https://doi.org/10.1016/j.colsurfb.2010.01.033
Halperim B, Beezzi G, Mene G et al (1951) Etude quantitative de l’activite granulo plexique du systeme reticulo-endothelial parl’plexique intravenuse d’encre dechine chez les diverses espces animals. Ann Inst Pasteure 80:582–604 PMID: 13093914
He H, Zou Z, Wang B et al (2020) Copper oxide nanoparticles induce oxidative dna damage and cell death via copper ion-mediated p38 mapk activation in vascular endothelial cells. Int J Nanomed 15:3291–3302. https://doi.org/10.2147/IJN.S241157
IBM Corp (2011) IBM SPSS statistics for windows, version 20.0. IBM Corp, Armonk
Johnson BM, Fraietta JA, Gracias DT (2015) Acute exposure to ZnO NPs induces autophagic immune cell death. Nanotoxicol 9(6):737–748. https://doi.org/10.3109/17435390.2014.974709
Kang M, Lim CH, Han JH (2013) Comparison of toxicity and deposition of nano-sized carbon black aerosol prepared with or without dispersing sonication. Toxicol Res 29(2):121–127. https://doi.org/10.5487/TR.2013.29.2.121
Karlsson HL, Gustafsson J, Cronholm P et al (2009) Size-dependent toxicity of metal oxide particles–a comparison between nano- and micrometer size. Toxicol Lett 188:112–118. https://doi.org/10.1016/2009.03.014
Li T, Kon N, Jiang L (2012) Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and science. Cell 149(6):1269–1283. https://doi.org/10.1016/j.cell.2012.04.026
Linic S, Aslam U, Boerigter M et al (2015) Photochemical transformations on plasmonic metal nanoparticles. Nat Mater 14:567–576. https://doi.org/10.1038/nmat4281
Masarudin MJ, Cutts SM, Evison BJ et al (2015) Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: application to the passive encapsulation of [(14)C]-doxorubicin. Nanotechn Sci Appl 8:67–80. https://doi.org/10.2147/NSA.S91785
Miron D, Mahbubeh S (2014) Acute effect of nano-copper on liver tissue and function in rat. Nanomed J 1(5):331–338
Mohammadyari A, Razavipour S, beige M et al (2014) Explore in-vivo toxicity assessment of copper oxide nanoparticle in Wistar rats. J Biol Todys world 3(6):124–128. https://doi.org/10.15412/J.JBTW
Murdock RC, Braydich-Stolle L, Schrand AM et al (2008) Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol Sci 101:239–253. https://doi.org/10.1093/toxsci/kfm240
Oberdörster G, Maynard A, Donaldson K et al (2005) Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol 2(1):8. https://doi.org/10.1186/1743-8977-2-8
Ohkawa H, Ohishi N, Yagi K et al (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Ou L, Song B, Liang H (2016) Toxicity of graphene-family nano- particles: a general review of the origins and mechanisms. Part Fibre Toxicol 13(1):57. https://doi.org/10.1186/s12989-016-0168-y
Pelegrino MT, Kohatsu MY, Seabra AB et al (2020) Effects of copper oxide nanoparticles on growth of lettuce)lactuca sativa l.) seedlings and possibleimplications of nitric oxide in their antioxidative defense. Environ Monit Asses 192:232. https://doi.org/10.1007/s10661-020-8188-3
Pietrofesa RA, Park K, Mishra OP et al (2021) Copper oxide nanoparticle-induced acute inflammatory response and injury in murine lung is ameliorated by synthetic secoisolariciresinol diglucoside (Lgm2605). Int J Mol Sci 22:9477. https://doi.org/10.3390/ijms22179477
Rabia AM, Azab EA, Karema EM et al (2019) Hepatotoxicity induced by copper oxide and zinc oxide nanoparticles and their mixtures in male albino rats. East Afr Scholars J Med Sci 2(11):650–660. https://doi.org/10.21203/rs.3.rs-218699/v1
Reynolds ES (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol 17:258. https://doi.org/10.1083/jcb.17.1.208
Roda E, Bottone MG, Biggiogera M et al (2019) Pulmonary and hepatic effects after low dose exposure to nano-silver: early and long-lasting histological and ultrastructural alterations in rat. Toxicology 6:1047–1060. https://doi.org/10.1016/j.toxrep.2019.09.008
Rossner PJ, Vrbova K, Rossnerova A et al (2020) Gene expression and epigenetic changes in mice following inhalation of copper(ii) oxide nanoparticles. Nanomaterials 10:550. https://doi.org/10.3390/nano10030550
Sadauskas E, Wallin H, Stoltenberg M et al (2007) Kupffer cells are central in the removal of nanoparticles from the organism. Part Fibre Toxicol 4(1):10. https://doi.org/10.1186/1743-8977-4-10
Schumann G, Klauke R (2003) New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: preliminary upper reference limits obtained in hospitalized subjects. Clin Chim Acta 327:69–79. https://doi.org/10.1016/s0009-8981(02)00341-8
Semisch A, Ohle J, Witt B et al (2014) Cytotoxicity and genotoxicity of nano-and microparticulate coppe roxide: role of solubility and intracellular bioavailability. Part Fibre Toxicol 11(1):10. https://doi.org/10.1186/1743-8977-11-10
Seo SD, Lee DH, Kim JC et al (2013) Room-temperature synthesis of CuO/graphene nanocomposite electrodes for high lithium storage capacity. Ceram Int 39(2):1749–1755. https://doi.org/10.1016/j.ceramint.08.021
Sharifi S, Behzadi S, Laurent S et al (2012) Toxicity of nanomaterials. Chem Soc Rev 41(6):2323–2343. https://doi.org/10.1039/c1cs15188f
Smith KR, Klei LR, Barchowsky A (2001) Arsenit stimulates plasma membrane NADPH oxidase in vascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 280(3):442–449. https://doi.org/10.1152/ajplung.2001.280.3.L442
Tulinska J, Mikusova ML, Liskova A et al (2022) Copper oxide nanoparticles stimulatethe immune response and decrease antioxidant defense in mice after six-week inhalation. Front Immunol 13: 874253. https://doi.org/10.3389/2022.874253
Valentini X, Rugira P, Frau A et al (2019) Hepatic and renal toxicity induced by TiO2 nanoparticles in rats: a morphological and metabonomic study. J Toxic 10:1155. https://doi.org/10.1155/2019/5767012
Vasilakes AL, Dziubla TD, Wattamwar PP (2013) Polymeric nanoparticles engineering polymer systems for improved drug delivery. Natl Libarery Med. https://doi.org/10.3390/gels4040080
Wang C, Zeng W, Zhang H et al (2014) Synthesis and growth mechanism of CuO nanostructures and their gas sensing properties. J Mater Sci 25:2041–2046. https://doi.org/10.1007/s10854-014-1837-y
Wang T, Bai J, Jiang X et al (2012) Cellular uptake of nanoparticles by membrane penetration: a study combining confocal microscopy with FTIR spectroelectrochemistry. ACS Nano 6(2):1251–1259. https://doi.org/10.1021/nn203892h
Xiaofeng L, Hu Z, Yong Z et al (2018) Intranasal delivery of copper oxide nanoparticles induces pulmonary toxicity and fibrosis in c57bl/6 mice. Sci Rep 8:4499. https://doi.org/10.1038/s41598-018-22556-7
Yallapu MM, Chauhan N, Othman SF (2015) Implications of protein corona on physico-chemical and biological properties of magnetic NPs. Biomaterials 46:1–12. https://doi.org/10.1016/j.biomaterials.2014.12.045
Yokohira M, Hashimoto N, Yamakawa K et al (2009) Lung carcinogenic bioassay of CuO and TiO2 nanoparticles with intratracheal instillation using F344 male rats. J Toxicol Pathol 22:71–78. https://doi.org/10.1293/tox.22.71
Zhou K, Wang R, Xu B et al (2006) Synthesis, characterization and catalytic properties of CuO nanocrystals with various shapes. Nanotechnology 17:3939–3943. https://doi.org/10.1088/0957-4484/17/15/055
Zou Y, Li Y, Zhang N et al (2011) Flower-like CuO synthesized by CTAB-assisted hydrothermal method. Bull Mater Sci 34(4):967–971. https://doi.org/10.1007/s12034-011-0223-0
Acknowledgements
The authors are thankful to STDF and EKB for supporting. The authors are grateful to Dr. Samaa.I. El-Dek., Head of Materials science and nanotechnology department Faculty of postgraduate studies for Advanced Sciences, Director of the international ranking office Beni-Suef University, BSU, Egypt. samaa@psas.bsu.edu.eg.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). None.
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly. AMA, ESA, MS and AAF, FKK and MA have contributed in suggesting the design of the work, preparation and analysis of the results, and interpretation of data and discussion. In addition, AMA has performed the practical part. All authors are in agreement with the contents of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
TThe authors have not disclosed any competing interests.
Ethical approval
All animal experiments should comply with the ARRIVE guidelines and should be carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) and Beni-Suef University (BSU-IACUC, No. 019–78).
Consent for publication
The manuscript is an original work and has not been submitted for publication in another journal. I also confirm that all the listed authors have participated actively in the study, and have seen and approved the submitted manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Azeem, A.M., Abdel-Rehiem, E.S., Farghali, A.A. et al. Comparative toxicological evaluations of novel forms nano-pesticides in liver and lung of albino rats. J Mol Histol 54, 157–172 (2023). https://doi.org/10.1007/s10735-023-10115-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-023-10115-y