Abstract
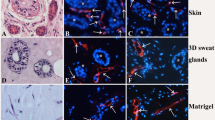
Victims with extensive and deep burns are unable to regenerate eccrine sweat glands. Combining of stem cells and biomimetic ECM to generate cell-based 3D tissues is showing promise for tissue repair and regeneration. We co-cultured BrdU-labeled bone marrow-derived mesenchymal stem cells (BM-MSCs) and eccrine sweat gland cells in Matrigel for 2 weeks in vitro and then evaluated for BM-MSCs differentiation into functional eccrine sweat gland cells by morphological assessment and immunohistochemical double staining for BrdU/pancytokeratin, BrdU/ZO-2, BrdU/E-cadherin, BrdU/desmoglein-2, BrdU/Na+–K+-ATPase α, BrdU/NHE1 and BrdU/CFTR. Cells formed spheroid-like structures in Matrigel, and BrdU-labeled BM-MSCs were involved in the 3D reconstitution of eccrine sweat gland tissues, and the incorporated BM-MSCs expressed an epithelial cell marker (pancytokeratin), epithelial cell junction proteins (ZO-2, E-cadherin and desmoglein-2) and functional proteins of eccrine sweat glands (Na+–K+-ATPase α, NHE1 and CFTR). In conclusion, three-dimensional co-culture of BM-MSCs and eccrine sweat gland cells in Matrigel promotes the transdifferentiation of BM-MSCs into potentially functional eccrine sweat gland cells.




Similar content being viewed by others
Abbreviations
- 3D:
-
Three-dimensional
- AEC:
-
3-Amino-9-ethylcarbazole
- BCIP/NBT:
-
5-Bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium
- BM-MSCs:
-
Bone marrow-derived mesenchymal stem cells
- BPE:
-
Bovine pituitary extract
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- DMEM/F12:
-
Dulbecco’s modified Eagle’s medium/Ham’s F12 (1:1)
- ECM:
-
Extracellular matrix
- EGF:
-
Epidermal growth factor
- HE:
-
Hematoxylin and eosin
- FGF:
-
Fibroblast growth factor
- IGF:
-
Insulin-like growth factor
- KSFM:
-
Keratinocyte serum-free medium
- Matrigel:
-
Matrigel basement membrane matrix
- MSCs:
-
Mesenchymal stem cells
- NHE1:
-
Sodium–hydrogen exchanger 1
- PBS:
-
Phosphate-buffered saline
- rhEGF:
-
Recombinant human epidermal growth factor
- TGF-β:
-
Transforming growth factor beta
References
Brown MB, Haack KK, Pollack BP, Millard-Stafford M, McCarty NA (2011) Low abundance of sweat duct Cl-channel CFTR in both healthy and cystic fibrosis athletes with exceptionally salty sweat during exercise. Am J Physiol Regul Integr Comp Physiol 300:R605–R615
Cheema FH, Polvani G, Argenziano M, Pesce M (2012) Combining stem cells and tissue engineering in cardiovascular repair—a step forward to derivation of novel implants with enhanced function and self-renewal characteristics. Recent Pat Cardiovasc Drug Discov 7:10–20
Cooke ME, Allon AA, Cheng T et al (2011) Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthr Cartil OARS Osteoarthr Res Soc 19:1210–1218
Deng W, Han Q, Liao L et al (2005) Engrafted bone marrow-derived flk-(1 +) mesenchymal stem cells regenerate skin tissue. Tissue Eng 11:110–119
Fu X, Li J, Sun X, Sun T, Sheng Z (2005a) Epidermal stem cells are the source of sweat glands in human fetal skin: evidence of synergetic development of stem cells, sweat glands, growth factors, and matrix metalloproteinases. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc 13:102–108
Fu X, Li X, Cheng B, Chen W, Sheng Z (2005b) Engineered growth factors and cutaneous wound healing: success and possible questions in the past 10 years. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc 13:122–130
Fu X, Qu Z, Sheng Z (2006) Potentiality of mesenchymal stem cells in regeneration of sweat glands. J Surg Res 136:204–208
Granger D, Marsolais M, Burry J, Laprade R (2003) Na+/H+ exchangers in the human eccrine sweat duct. Am J Physiol Cell Physiol 285:C1047–C1058
Kleinman HK, Martin GR (2005) Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol 15:378–386
Li H, Fu X, Ouyang Y, Cai C, Wang J, Sun T (2006) Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res 326:725–736
Li H, Chen L, Zhang M, Tang S, Fu X (2013) Three-dimensional culture and identification of human eccrine sweat glands in Matrigel basement membrane matrix. Cell Tissue Res 354:897–902
Li H, Zhang X, Zeng S et al (2014) The cellular localization of Na(+)/H(+) exchanger 1, cystic fibrosis transmembrane conductance regulator, potassium channel, epithelial sodium channel gamma and vacuolar-type H+-ATPase in human eccrine sweat glands. Acta Histochem 116:1237–1243
Li H, Chen L, Zeng S et al (2015) Matrigel basement membrane matrix induces eccrine sweat gland cells to reconstitute sweat gland-like structures in nude mice. Exp Cell Res 332:67–77
Liu X, Zhang Z, Yan X et al (2014a) The Rho kinase inhibitor Y-27632 facilitates the differentiation of bone marrow mesenchymal stem cells. J Mol Histol 45:707–714
Liu X, Zuo D, Fan H et al (2014b) Over-expression of CXCR4 on mesenchymal stem cells protect against experimental colitis via immunomodulatory functions in impaired tissue. J Mol Histol 45:181–193
Lu CP, Polak L, Rocha AS et al (2012) Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 150:136–150
Maria OM, Maria O, Liu Y, Komarova SV, Tran SD (2011) Matrigel improves functional properties of human submandibular salivary gland cell line. Int J Biochem Cell Biol 43:622–631
Mohd Hilmi AB, Halim AS (2015) Vital roles of stem cells and biomaterials in skin tissue engineering. World J Stem Cells 7:428–436
Nejsum LN, Praetorius J, Nielsen S (2005) NKCC1 and NHE1 are abundantly expressed in the basolateral plasma membrane of secretory coil cells in rat, mouse, and human sweat glands. Am J Physiol Cell Physiol 289:C333–C340
Pal A, Kleer CG (2014) Three dimensional cultures: a tool to study normal acinar architecture vs. malignant transformation of breast cells. J Vis Exp. doi:10.3791/51311
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Reddy MM, Quinton PM (2003) Functional interaction of CFTR and ENaC in sweat glands. Pflugers Arch 445:499–503
Sheng Z, Fu X, Cai S et al (2009) Regeneration of functional sweat gland-like structures by transplanted differentiated bone marrow mesenchymal stem cells. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc 17:427–435
Silva GV, Litovsky S, Assad JA et al (2005) Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 111:150–156
St Johnston D, Ahringer J (2010) Cell polarity in eggs and epithelia: parallels and diversity. Cell 141:757–774
Sun J, Zhang T, Zhang P et al (2014) Overexpression of the PLAP-1 gene inhibits the differentiation of BMSCs into osteoblast-like cells. J Mol Histol 45:599–608
Takashi H, Katsumi M, Toshihiro A (2007) Hepatocytes maintain their function on basement membrane formed by epithelial cells. Biochem Biophys Res Commun 359:151–156
Tang-Schomer MD, White JD, Tien LW et al (2014) Bioengineered functional brain-like cortical tissue. Proc Natl Acad Sci USA 111:13811–13816
Wang Y, Liu ZY, Zhao Q, Sun TZ, Ma K, Fu XB (2013) Future application of hair follicle stem cells: capable in differentiation into sweat gland cells. Chin Med J 126:3545–3552
Wang Y, Yin Y, Jiang F, Chen N (2015) Human amnion mesenchymal stem cells promote proliferation and osteogenic differentiation in human bone marrow mesenchymal stem cells. J Mol Histol 46:13–20
Zhang CP, Fu XB (2008) Therapeutic potential of stem cells in skin repair and regeneration. Chin J Traumatol 11:209–221
Zhang Z, Gupte MJ, Ma PX (2013) Biomaterials and stem cells for tissue engineering. Expert Opin Biol Ther 13:527–540
Zhang M, Zeng S, Zhang L et al (2014) Localization of Na(+)-K(+)-ATPase α/β, Na(+)-K(+)-2Cl-cotransporter 1 and aquaporin-5 in human eccrine sweat glands. Acta Histochem 116:1374–1381
Zhang C, Chen Y, Fu X (2015) Sweat gland regeneration after burn injury: is stem cell therapy a new hope? Cytotherapy 17:526–535
Zonana J, Elder ME, Schneider LC et al (2000) A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO). Am J Hum Genet 67:1555–1562
Acknowledgments
The manuscript was supported in part by the National Natural Science Foundation of China (81071551, 81471882) and the Natural Science Foundation of Guangdong Province (2014A030313476).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare we have no competing financial, personal or other relationships with other people or organizations.
Rights and permissions
About this article
Cite this article
Li, H., Li, X., Zhang, M. et al. Three-dimensional co-culture of BM-MSCs and eccrine sweat gland cells in Matrigel promotes transdifferentiation of BM-MSCs. J Mol Hist 46, 431–438 (2015). https://doi.org/10.1007/s10735-015-9632-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-015-9632-5