Abstract
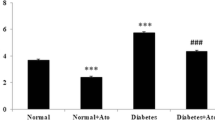
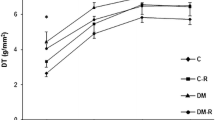
Diabetic hazard on the myocardium is a complication of diabetes that intensifies its morbidity and increases its mortality. Therefore, alleviation of diabetic cardiomyopathy (DCM) by a reliable drug remains a matter of interest in experimental research. The aim of this study was to explore the structural alterations in the myocardium induced by atorvastatin (ATOR) in DCM, induced by streptozotocin (STZ), along with the associated changes occurring in apoptosis and oxidative stress markers. Thirty-two rats were divided into four groups; group A (control), group B (non-diabetic, received ATOR, orally, 50 mg/kg daily), group C (DCM, received STZ 70 mg/kg, single i.p. injection) and group D (DCM + ATOR). After 6 weeks, left ventricle (LV) specimens were prepared for histological and immunohistochemical study by hematoxlyin and eosin, Masson`s trichrome, anti-cleaved caspase-3 stains as well as for assays of oxidative stress markers. All data were measured morphometrically and statistically analyzed. The DCM group showed disorganization of the cardiomyocytes, interstitial edema, numerous fibroblasts, significant increases in the collagen volume fraction (p < 0.001), cleaved caspase-3 expression % area (p < 0.001) and, malondialdehyde in blood (p < 0.001), in LV (p < 0.05) compared with DCM + ATOR group. The latter has LV wall thickness, relative heart weight and antioxidant activities nearly similar to the control, independent from ATOR lipid-lowering effect. Therefore, ATOR can preserve myocardial structure in DCM nearly similar to normal. This may be achieved by suppressing apoptosis that parallels the correction of the antioxidant markers, which can be considered as non-lipid lowering benefit of statins.





Similar content being viewed by others
References
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Burgess ML, McCrea JC, Hedrick HL (2001) Age-associated changes in cardiac matrix and integrins. Mech Ageing Dev 122:1739–1756
Cai L, Li W, Wang G et al (2002) Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes 51:1938–1948
Cai L, Wang Y, Zhou G et al (2006) Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol 48:1688–1697
Chatterjee S, Stewart AS, Bish LT et al (2002) Viral gene transfer of the antiapoptotic factor Bcl-2 protects against chronic postischemic heart failure. Circulation 106:I-212–I-217
Chen B, Zhang Y, Liu G et al (2008) Effects of valsartan, mycophenolate mofetil and their combined application on TRAIL and nuclear factor-kappaB expression in the kidneys of diabetic rats. Zhonghua yi xue za zhi 88:540–545
Chen YH, Feng B, Chen ZW (2012) Statins for primary prevention of cardiovascular and cerebrovascular events in diabetic patients without established cardiovascular diseases: a meta-analysis. Exp Clin Endocrinol Diabetes 120:116–120
Condorelli G, Roncarati R, Ross J et al (2001) Heart-targeted overexpression of caspase3 in mice increases infarct size and depresses cardiac function. Proc Natl Acad Sci USA 98:9977–9982
Dai Q-M, Lu J, Liu N-F (2011) Fluvastatin attenuates myocardial interstitial fibrosis and cardiac dysfunction in diabetic rats by inhibiting over-expression of connective tissue growth factor. Chin Med J (Engl) 124:89–94
Danilova IG, Sarapultsev PA, Medvedeva SU et al (2015) Morphological restructuring of myocardium during the early phase of experimental diabetes mellitus. Anat Rec (Hoboken, NJ: 2007) 298:396–407
Duncan JG (2011) Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim Biophys Acta 1813:1351–1359
Falcao-Pires I, Leite-Moreira AF (2012) Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev 17:325–344
Galvez AS, Ulloa JA, Chiong M et al (2003) Aldose reductase induced by hyperosmotic stress mediates cardiomyocyte apoptosis: differential effects of sorbitol and mannitol. J Biol Chem 278:38484–38494
Gawai KR, Pawar SS (1984) Purification and characterization of glutathione-S-transferase from liver cytosol of phenobarbital-treated rabbits. Xenobiotica 14:605–607
Gawlowski T, Stratmann B, Stork I et al (2009) Heat shock protein 27 modification is increased in the human diabetic failing heart. Horm Metab Res 41:594–599
Guleria RS, Singh AB, Nizamutdinova IT et al (2013) Activation of retinoid receptor-mediated signaling ameliorates diabetes-induced cardiac dysfunction in Zucker diabetic rats. J Mol Cell Cardiol 57:106–118
Hamblin M, Smith HM, Hill MF (2007) Dietary supplementation with vitamin E ameliorates cardiac failure in type I diabetic cardiomyopathy by suppressing myocardial generation of 8-iso-prostaglandin F2alpha and oxidized glutathione. J Card Fail 13:884–892
Huang YQ, Wang X, Kong W (2010) Diabetic cardiomyopathy. Sheng li ke xue jin zhan [Progress in physiology] 41:31–36
Huynh K, Bernardo BC, McMullen JR et al (2014) Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther 142:375–415
Konduracka E, Cieslik G, Galicka-Latala D et al (2013) Myocardial dysfunction and chronic heart failure in patients with long-lasting type 1 diabetes: a 7-year prospective cohort study. Acta Diabetol 50:597–606
Lim SH, Lee J (2012) Methanol extract of Cassia mimosoides var. nomame attenuates myocardial injury by inhibition of apoptosis in a rat model of ischemia-reperfusion. Prev Nutr Food Sci 17:177–183
Lim SH, Kim MY, Lee J (2014) Apple pectin, a dietary fiber, ameliorates myocardial injury by inhibiting apoptosis in a rat model of ischemia/reperfusion. Nutr Res Pract 8:391–397
Liu YS, Huang ZW, Wang L et al (2015) Sitagliptin alleviated myocardial remodeling of the left ventricle and improved cardiac diastolic dysfunction in diabetic rats. J Pharmacol Sci 127:260–274
Long WK, Carson PE (1961) Increased erythrocyte glutathione reductase activity in diabetes mellitus. Biochem Biophys Res Commun 5:394–399
Mano Y, Anzai T, Kaneko H et al (2011) Overexpression of human C-reactive protein exacerbates left ventricular remodeling in diabetic cardiomyopathy. Circ J 75:1717–1727
Miao Y, Zhang W, Zhong M et al (2007) Activation of transforming growth factor-beta1/Smads signal pathway in diabetic cardiomyopathy and effects of valsartan thereon: experiment with rats. Zhonghua yi xue za zhi 87:366–370
Narula J, Pandey P, Arbustini E et al (1999) Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci USA 96:8144–8149
Ni Q, Wang J, Li EQ et al (2011) Study on the protective effect of shengmai san (see text) on the myocardium in the type 2 diabetic cardiomyopathy model rat. Journal of traditional Chinese medicine = Chung i tsa chih ying wen pan/sponsored by All-China Association of Traditional Chinese Medicine. Acad Tradit Chin Med 31:209–219
Ouyang C, You J, Xie Z (2014) The interplay between autophagy and apoptosis in the diabetic heart. J Mol Cell Cardiol 71:71–80
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Palomer X, Salvado L, Barroso E et al (2013) An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int J Cardiol 168:3160–3172
Pappachan JM, Varughese GI, Sriraman R et al (2013) Diabetic cardiomyopathy: pathophysiology, diagnostic evaluation and management. World J Diabetes 4:177–189
Petrova R, Yamamoto Y, Muraki K et al (2002) Advanced glycation endproduct-induced calcium handling impairment in mouse cardiac myocytes. J Mol Cell Cardiol 34:1425–1431
Philipp S, Pagel I, Hohnel K et al (2004) Regulation of caspase 3 and Fas in pressure overload-induced left ventricular dysfunction. Eur J Heart Fail 6:845–851
Picatoste B, Ramirez E, Caro-Vadillo A et al (2013) Sitagliptin reduces cardiac apoptosis, hypertrophy and fibrosis primarily by insulin-dependent mechanisms in experimental type-II diabetes. Potential roles of GLP-1 isoforms. PLoS ONE 8:e78330
Ramirez E, Klett-Mingo M, Ares-Carrasco S et al (2013) Eplerenone attenuated cardiac steatosis, apoptosis and diastolic dysfunction in experimental type-II diabetes. Cardiovasc Diabetol 12:172
Scheubel RJ, Bartling B, Simm A et al (2002) Apoptotic pathway activation from mitochondria and death receptors without caspase-3 cleavage in failing human myocardium: fragile balance of myocyte survival? J Am Coll Cardiol 39:481–488
Schilling JD, Mann DL (2012) Diabetic cardiomyopathy: bench to bedside. Heart Fail Clin 8:619–631
Shida T, Nozawa T, Sobajima M et al (2014) Fluvastatin-induced reduction of oxidative stress ameliorates diabetic cardiomyopathy in association with improving coronary microvasculature. Heart Vessels 29:532–541
Sun M, Dawood F, Wen WH et al (2004) Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation 110:3221–3228
Tarquini R, Lazzeri C, Pala L et al (2011) The diabetic cardiomyopathy. Acta Diabetol 48:173–181
Taylor F, Huffman MD, Macedo AF et al (2013) Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 1:Cd004816
Tietz NW, Burtis CA, Ashwood ER (2006) Tietz textbook of clinical chemistry and molecular diagnostics. Elsevier, St. Louis
Van Linthout S, Riad A, Dhayat N et al (2007) Anti-inflammatory effects of atorvastatin improve left ventricular function in experimental diabetic cardiomyopathy. Diabetologia 50:1977–1986
Wang YJ, Fu GS, Chen FM et al (2009) The effect of valsartan and fluvastatin on the connective tissue growth factor expression in experimental diabetic cardiomyopathy. Zhonghua nei ke za zhi 48:660–665
Xie Z, He C, Zou MH (2011) AMP-activated protein kinase modulates cardiac autophagy in diabetic cardiomyopathy. Autophagy 7:1254–1255
Xue D, Shaham S, Horvitz HR (1996) The Caenorhabditis elegans cell-death protein CED-3 is a cysteine protease with substrate specificities similar to those of the human CPP32 protease. Genes Dev 10:1073–1083
Zhang YL, Wei JR (2013) 3-nitrotyrosine, a biomarker for cardiomyocyte apoptosis induced by diabetic cardiomyopathy in a rat model. Mol Med Rep 8:989–994
Zhong M, Zhang Y, Miao Y et al (2006) Mechanism of reversion of myocardial interstitial fibrosis in diabetic cardiomyopathy by valsartan. Zhonghua yi xue za zhi 86:232–236
Zidar N, Dolenc-Strazar Z, Jeruc J et al (2006) Immunohistochemical expression of activated caspase-3 in human myocardial infarction. Virchows Arch Int J Pathol 448:75–79
Zou MH, Xie Z (2013) Regulation of interplay between autophagy and apoptosis in the diabetic heart: new role of AMPK. Autophagy 9:624–625
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Hamid, A.A.M., Firgany, A.ED.L. Atorvastatin alleviates experimental diabetic cardiomyopathy by suppressing apoptosis and oxidative stress. J Mol Hist 46, 337–345 (2015). https://doi.org/10.1007/s10735-015-9625-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-015-9625-4