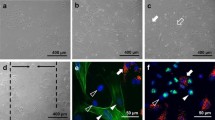
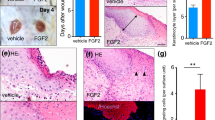
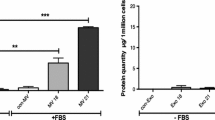
Abstract
The adequate reconstitution of human soft tissue wounds requires the coordinated interaction of endothelial cells and fibroblasts during the proliferation phase of healing. Endothelial cells assure neoangiogenesis, fibroblasts fill the defect and provide extracellular matrix proteins, and myofibroblasts are believed to support the reconstitution of microvessels. In the present study, we combined in vitro-wound size measurement and multicolour immunocytochemical staining of co-cultured human dermal microvascular endothelial cells and normal human dermal fibroblasts, recently introduced as co-culture scratch-wound migration assay. Applying antibodies for α-smooth-muscle actin, von Willebrand factor, extra domain A fibronectin and endothelin-1, we were able to monitor proliferation, migration and the differentiation process from fibroblasts to myofibroblasts as a response to hypoxia. Furthermore, we verified, whether transforming growth factor β1 (TGFβ1) and endothelin-1 are able to mediate this response. We show, that proliferation and migration of endothelial cells and fibroblasts decreased under hypoxia. The additional administration of TGFβ1 did not significantly attenuate this decrease. Solely the myofibroblast population in co-culture adapted well to hypoxia, when cultures were supplemented with TGFβ1. Considerating the data concerning TGFβ1 and endothelin-1, we propose a model explaining the cellular interaction during early and late proliferation phase of human wound healing.







Similar content being viewed by others
Abbreviations
- α-SMA:
-
α-Smooth-muscle actin
- bFGF:
-
Basic fibroblast growth factor
- CCSWMA:
-
Co-culture scratch-wound migration assay
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- ECM:
-
Extracellular matrix
- ED-A-fn:
-
Extra domain A fibronectin
- ET-1:
-
Endothelin-1
- HDMEC:
-
Human dermal microvascular endothelial cell/s
- NHDF:
-
Normal human dermal fibroblast/s
- MF:
-
Myofibroblast/s
- sd:
-
Standard deviation
- TGFβ1 :
-
Transforming growth factor β1
- vWF:
-
von Willebrand factor
References
Amadeu TP, Coulomb B, Desmouliere A, Costa AMA (2003) Cutaneous wound healing: myofibroblastic differentiation and in vitro models. Int J Low Extrem Wounds 2(2):60–68
Basilico N, Speciale L, Parapini S, Ferrante P, Taramelli D (2002) Endothelin-1 production by a microvascular endothelial cell line treated with Plasmodium falciparum parasitized red blood cells. Clin Sci 103(48):464–466
Brunner G, Blakytny R (2004) Extracellular regulation of TGF-ß activity in wound repair: growth factor latency as a sensor mechanism for injury. Thromb Haemost 92:253–261
Chen CP, Yang YC, Su TH, Chen CY, Aplin JD (2005) Hypoxia and transforming growth factor-β1 act independently to increase extracellular matrix production by placental fibroblasts. J Clin Endocrinol Metab 90:1083–1090
Chin GA, Diegelmann RF, Schultz GS (2005) Cellular and molecular regulation of wound healing. Wound Healing 2005:17–39
Cordeiro MF, Bhattacharya SS, Schultz GS, Khaw PT (2000) TGF-β1, -β2, and -β3 in vitro: biphasic effects on tenon’s fibroblast contraction, proliferation and migration. Invest Ophthalmol Vis Sci 41:756–763
Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G (1993) Transforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122:103–111
Desmouliere A, Redard M, Darby I, Gabbiani G (1995) Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146(1):56–66
Diegelmann RF, Evans MC (2004) Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 9:283–289
Dube J, Chakir J, Dube C, Grimard Y, Laviolette M, Boulet LP (2000) Synergistic action of endothelin (ET)-1 on the activation of bronchial fibroblast isolated from normal and asthmatic subjects. Int J Exp Path 81:429–437
Ellis I, Banyard J, Schor SL (1997) Differential response of fetal and adult fibroblasts to cytokines: cell migration and hyaluronan synthesis. Development 124:1593–1600
Eul B, Rose F, Krick S, Savai R, Goyal P, Klepetko W, Grimminger F, Weissmann N, Seeger W, Hänze J (2006) Impact of HIF-1α and HIF-2α on proliferation and migration of human pulmonary artery fibroblasts in hypoxia. FASEB J 20(1):163–165
Gabbiani G (2003) The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 200(4):500–503
Gallelli L, Pelaia G, D’Agostino B, Cuda G, Vatrella A, Fratto D, Gioffre V, Galderisi U, De Nardo M, Mastruzzo C, Salinaro ET, Maniscalco M, Sofia M, Crimi N, Rossi F, Caputi M, Costanzo FS, Maselli R, Marsico SA, Cancheri C (2005) Endothelin-1 induces proliferation of human lung fibroblasts and IL-11 secretion through an ET(A) receptor-dependent activation of MAP kinases. J Cell Biochem 96(4):858–68
Gharaee-Kermani M, Phan SH (2001) Role of cytokines and cytokine therapy in wound healing and fibrotic diseases. Curr Pharm Des 7:1083–1103
Hogg N, Browning J, Howard T, Winterford C, Fitzpatrick D, Gobe G (1999) Apoptosis in vascular endothelial cells caused by serum deprivation, oxidative stress and transforming growth factor-beta. Endothelium 7(1):35–49
Iruela-Arispe ML, Sage EH (1993) Endothelial cells exhibiting angiogenesis in vitro proliferate in response to TGF-beta 1. J Cell Biochem 52(4):414–430
Kernochan LE, Tran BN, Tangkijvanich P, Melton AC, Tam SP, Yee HF (2002) Endothelin-1 stimulates human colonic myofibroblast contraction and migration. Gut 50:65–70
Kourembanas S, Marsden PA, McQuillan LP, Faller DV (1991) Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest 88:1054–1057
Li W, Li Y, Guan S, Fan J, Cheng CF, Bright AM, Chinn C, Chen M, Woodley DT (2007) Extracellular heat shock protein-90a: linking hypoxia to skin cell motility and wound healing. The EMBO Journal 2007:1–13
Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S (1996) Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci USA 93(9):4219–4223
Merwin JR, Newman W, Beall LD, Tucker A, Madri J (1991) Vascular cells respond differentially to transforming growth factors beta 1 and beta 2 in vitro. Am J Pathol 138(1):37–51
Minchenko A, Caro J (2000) Regulation of endothelin-1 gene expression in human microvascular endothelial cells by hypoxia and cobalt: Role of hypoxia responsive element. Mol Cell Biochem 208:53–62
Mogford JE, Tawil N, Chen A, Gies D, Xia Y, Mustoe TA (2002) Effect of age and hypoxia on TGFß1 receptor expression and signal transduction in human dermal fibroblasts: impact on cell migration. J Cell Physiol 190:259–265
Moulin V, Castilloux G, Auger FA, Garrel D, O`Connor-McCourt MD, Germain L (1998) Modulated response to cytokines of human wound healing myofibroblasts compared to dermal fibroblasts. Exp Cell Res 238:283–293
Oberringer M, Jennewein M, Motsch SE, Pohlemann T, Seekamp A (2005) Different cell cycle responses of wound healing protagonists to transient in vitro hypoxia. Histochem Cell Biol 123(6):595–603
Oberringer M, Meins C, Bubel M, Pohlemann T (2007) A new in vitro wound model based on the co-culture of human dermal microvascular endothelial cells and human dermal fibroblasts. Biol Cell 99(4):197–207
Petzelbauer E, Springhorn JP, Tucker AM, Madri JA (1996) Role of plasminogen activator inhibitor in the reciprocal regulation of bovine aortic endothelial and smooth muscle cell migration by TGF-beta 1. Am J Pathol 149(3):923–931
Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB (1999) Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol 277:C1–C19
Ronnov-Jessen L, Petersen OW (1993) Induction of α-smooth muscle actin by transforming growth factor-β1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasis. Lab Invest 68:696–707
Saito T, Itoh H, Chun T, Igaki T, Mori Y, Yamashita J, Doi K, Tanaka T, Inoue M, Masatsugu K, Fukunaga Y, Sawada N, Tojo K, Saito Y, Hosoya T, Nakao K (1998) Oxidative stress suppresses the endothelial secretion of endothelin. J Cardiovasc Pharmacol 31(Suppl 1):345–347
Sakuda H, Nakashima Y, Kuriyama S, Sueishi K (1992) Media conditioned by smooth muscle cells cultured in a variety of hypoxic environments stimulates in vitro angiogenesis. A relationship to transforming growth factor-beta 1. Am J Pathol 141:1507–1516
Sankar S, Mahooti-Brooks N, Bensen L, McCarthy TL, Centrella M, Madri JA (1996) Modulation of transforming growth factor beta receptor levels on microvascular endothelial cells during in vitro angiogenesis. J Clin Invest 97(6):1436–1446
Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G (1998) The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol 142(3):873–881
Serini G, Gabbiani G (1999) Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res 250:273–283
Shephard P, Hinz B, Smola-Hess S, Meister JJ, Krieg T, Smola H (2004) Dissecting the roles of endothelin, TGF-ß and GM-CSF on myofibroblast differentiation by keratinocytes. Thromb Haemost 92:262–274
Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM, Leask A, Abraham DJ (2004) Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell 15:2707–2719
Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G (1986) A monoclonal antibody against α-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 103:2787–2796
Sun G, Stacey M, Bellini A, Marini M, Mattoli S (1997) Endothelin-1 induces bronchial myofibroblast differentiation. Peptides 18(9):1449–1451
Thiemermann C, Corder R (1992) Is endothelin-1 the regulator of myofibroblast contraction during wound healing?. Lab Invest 67(6):667–669
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechanoregulation of connective tissue remodelling. Mol Cell Biol 3:349–363
Trabold O, Wagner S, Wicke C, Scheuenstuhl H, Hussain Z, Rosen N, Seremetiev A, Becker HD, Hunt TK (2003) Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Rep Reg 11:504–509
Zhao L, Eghbali-Webb M (2001) Release of pro- and anti-angiogenic factors by human cardiac fibroblasts: effects on DNA synthesis and protection under hypoxia in human endothelial cells. Biochim Biophys Acta 1538(2–3):273–82
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oberringer, M., Meins, C., Bubel, M. et al. In vitro wounding: effects of hypoxia and transforming growth factor β1 on proliferation, migration and myofibroblastic differentiation in an endothelial cell-fibroblast co-culture model. J Mol Hist 39, 37–47 (2008). https://doi.org/10.1007/s10735-007-9124-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-007-9124-3