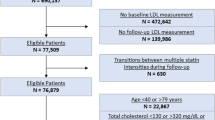
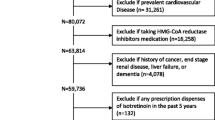
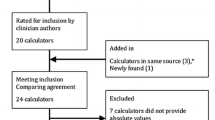
Abstract
Atherosclerotic cardiovascular disease (ASCVD) is among the leading causes of death in the US. Although research has shown that ASCVD has genetic elements, the understanding of how genetic testing influences its prevention and treatment has been limited. To this end, we model the health trajectory of patients stochastically and determine treatment and testing decisions simultaneously. Since the cholesterol level of patients is one controllable risk factor for ASCVD events, we model cholesterol treatment plans as Markov decision processes. We determine whether and when patients should receive a genetic test using value of information analysis. By simulating the health trajectory of over 64 million adult patients, we find that 6.73 million patients undergo genetic testing. The optimal treatment plans informed with clinical and genetic information save 5,487 more quality-adjusted life-years while costing $1.18 billion less than the optimal treatment plans informed with clinical information only. As precision medicine becomes increasingly important, understanding the impact of genetic information becomes essential.







Similar content being viewed by others
References
Kathiresan S, Srivastava D (2012) Genetics of human cardiovascular disease. Cell 148 (6):1242–1257. https://doi.org/10.1016/j.cell.2012.03.001
The CARDIoGRAMplusC4D Consortium (2013) Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Gen 45(1):25–33. https://doi.org/10.1038/ng.2480
Abraham G, Kowalczyk A, Zobel J, Inouye M (2013) Performance and Robustness of Penalized and Unpenalized Methods for Genetic Prediction of Complex Human Disease. Genet Epidemiol 37 (2):184–195. https://doi.org/10.1002/gepi.21698
Abraham G, Tye-Din J A, Bhalala O G, Kowalczyk A, Zobel J, Inouye M (2014) Accurate and Robust Genomic Prediction of Celiac Disease Using Statistical Learning. PLoS Genet 10(2):e1004137. https://doi.org/10.1371/journal.pgen.1004137, 1301.5948
Sun Y, Goodison S, Li J, Liu L, Farmerie W (2007) Improved breast cancer prognosis through the combination of clinical and genetic markers. Bioinformatics 23(1):30–37. https://doi.org/10.1093/bioinformatics/btl543
Vasan R S (2006) Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation 113(19):2335–2362. https://doi.org/10.1161/CIRCULATIONAHA.104.482570
Mega J L, Stitziel N O, Smith J G, Chasman D I, Caulfield M J, Devlin J J, Nordio F, Hyde C L, Cannon C P, Sacks F M, Poulter N R, Sever P S, Ridker P M, Braunwald E, Melander O, Kathiresan S, Sabatine M S (2015) Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet 385(9984):2264–2271. https://doi.org/10.1016/S0140-6736(14)61730-X
Bibbins-Domingo K, Grossman D C, Curry S J, Davidson K W, Epling J W, García F A R, Gillman M W, Kemper A R, Krist A H, Kurth A E, Landefeld C S, LeFevre M L, Mangione C M, Phillips W R, Owens D K, Phipps M G, Pignone M P (2016) Statin use for the primary prevention of cardiovascular disease in adults. J Am Med Assoc 316(19):1997–2007. https://doi.org/10.1001/jama.2016.15450
Goff D C, Lloyd-Jones D M, Bennett G, Coady S, D’Agostino R B, Gibbons R, Greenland P, Lackland D T, Levy D, O’Donnell C J, Robinson J G, Schwartz J S, Shero S T, Smith S C, Sorlie P, Stone N J, Wilson P WF (2014) 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation 129(25 SUPPL. 1). https://doi.org/10.1161/01.cir.0000437741.48606.98
Heron M (2018) Deaths: leading causes for 2016. Natl Vital Stat Rep 67 (6):1–77. http://www.ncbi.nlm.nih.gov/pubmed/30248017
Benjamin E J, Virani S S, Callaway C W, Chamberlain A M, Chang A R, Cheng S, Chiuve S E, Cushman M, Delling F N, Deo R, de Ferranti S D, Ferguson J F, Fornage M, Gillespie C, Isasi C R, Jiménez M C, Jordan L C, Judd S E, Lackland D, Lichtman J H, Lisabeth L, Liu S, Longenecker C T, Lutsey P L, Mackey J S, Matchar D B, Matsushita K, Mussolino M E, Nasir K, O’Flaherty M, Palaniappan L P, Pandey A, Pandey D K, Reeves M J, Ritchey M D, Rodriguez C J, Roth G A, Rosamond W D, Sampson U KA, Satou G M, Shah S H, Spartano N L, Tirschwell D L, Tsao C W, Voeks J H, Willey J Z, Wilkins J T, Wu Jason HY, Alger H M, Wong S S, Muntner P (2018) Heart disease and stroke statistics - 2018 update: a report from the American Heart Association. Circulation 137(12):E67–E492. https://doi.org/10.1161/CIR.0000000000000558. NIHMS150003
MacRae C A, Vasan R S (2016) The future of genetics and genomics. Circulation 133 (25):2634–2639. https://doi.org/10.1161/CIRCULATIONAHA.116.022547
Jarmul J, Pletcher M J, Hassmiller Lich K, Wheeler S B, Weinberger M, Avery C L, Jonas D E, Earnshaw S, Pignone M (2018) Cardiovascular genetic risk testing for targeting statin therapy in the primary prevention of atherosclerotic cardiovascular disease. Circ Cardiovascul Qual Outcome 11(4):e004171. https://doi.org/10.1161/CIRCOUTCOMES.117.004171
Khera A V, Emdin C A, Drake I, Natarajan P, Bick A G, Cook N R, Chasman D I, Baber U, Mehran R, Rader D J, Fuster V, Boerwinkle E, Melander O, Orho-Melander M, Ridker P M, Kathiresan S (2016) Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med 375(24):2349–2358. https://doi.org/10.1056/NEJMoa1605086
Natarajan P, Young R, Stitziel N O, Padmanabhan S, Baber U, Mehran R, Sartori S, Fuster V, Reilly D F, Butterworth A, Rader D J, Ford I, Sattar N, Kathiresan S (2017) Polygenic risk score identifies subgroup with higher burden of atherosclerosis and greater relative benefit from statin therapy in the primary prevention setting. Circulation 135(22):2091–2101. https://doi.org/10.1161/CIRCULATIONAHA.116.024436
Lewis C M, Vassos E (2020) Polygenic risk scores: From research tools to clinical instruments. Genome Med 12(1):1–11. https://doi.org/10.1186/s13073-020-00742-5
Knowles J W, Ashley E A (2018) Cardiovascular disease: The rise of the genetic risk score. PLoS Med 15(3):1–7. https://doi.org/10.1371/journal.pmed.1002546
Connor M J-, Bch M B, Natarajan P (2020) Current Clinical Implications of Coronary Artery Disease Polygenic Risk Scoring, pp 4–11
Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith G D, DeMets D, Evans S, Law M, MacMahon S, Martin S, Neal B, Poulter N, Preiss D, Ridker P, Roberts I, Rodgers A, Sandercock P, Schulz K, Sever P, Simes J, Smeeth L, Wald N, Yusuf S, Peto R (2016) Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 388(10059):2532–2561. https://doi.org/10.1016/S0140-6736(16)31357-5
Stone N J, Robinson J G, Lichtenstein A H, Bairey Merz C N, Blum C B, Eckel R H, Goldberg A C, Gordon D, Levy D, Lloyd-Jones D M, McBride P, Schwartz J S, Shero S T, Smith S C, Watson K, Wilson P W F (2014) 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation 129(25 suppl 2):S1–S45. https://doi.org/10.1161/01.cir.0000437738.63853.7a
Sussman J B, Wiitala W L, Zawistowski M, Hofer T P, Bentley D, Hayward R A (2017) The Veterans Affairs cardiac risk score: recalibrating the atherosclerotic cardiovascular disease score for applied use. Med Care 55(9):864–870. https://doi.org/10.1097/MLR.0000000000000781
Puterman M L (2014) Markov decision processes: discrete stochastic dynamic programming. Wiley
Raiffa H, Schlaifer R (1961) Applied statistical decision theory. Harvard University and MIT Press
Glover MJ, Jones E, Masconi KL, Sweeting MJ, Thompson SG, Powell JT, Ulug P, Bown MJ (2018) Discrete event simulation for decision modeling in health care: lessons from abdominal aortic aneurysm screening. Med Dec Making 38(4):439–451. https://doi.org/10.1177/0272989X17753380
Cipriano L E, Weber T A (2018) Population-level intervention and information collection in dynamic healthcare policy. Health Care Manag Sci 21(4):604–631. https://doi.org/10.1007/s10729-017-9415-5
Yokota F, Thompson K M (2004) Value of information literature analysis: a review of applications in health risk management. Med Dec Making 24(3):287–298. https://doi.org/10.1177/0272989X04263157
Ozcan Y A (2005) Quantitative methods in health care management: techniques and applications. Jossey-Bass.https://www.wiley.com/en-us/Quantitative+Methods+in+Health+Care+Management%3A+Techniques+and+Applications-p-9780787981341
Steuten L, Van De Wetering G, Groothuis-Oudshoorn K, Retèl V (2013) A systematic and critical review of the evolving methods and applications of value of information in academia and practice. PharmacoEconomics 31(1):25–48. https://doi.org/10.1007/s40273-012-0008-3
Heath A, Manolopoulou I, Baio G (2017) A review of methods for analysis of the expected value of information. Med Dec Making 37(7):747–758. https://doi.org/10.1177/0272989X17697692
Thompson KM, Yokota F (2004) Value of information analysis in environmental health risk management decisions: Past, present, and future. Risk Anal 24(3):635–650
Claxton K, Neumann P J, Araki S, Weinstein M C (2001) Bayesian value-of-information analysis: an application to a policy model of Alzheimer’s disease. Int J Technol Assess Health Care 17(1):38–55. https://doi.org/10.1017/S0266462301104058
Felli J C, Hazen G B (1998) Sensitivity analysis and the expected value of perfect information. Med Dec Making 18(1):95–109. https://doi.org/10.1177/0272989X9801800117
Felli J C, Hazen G B (1999) A Bayesian approach to sensitivity analysis. Health Econ 8 (3):263–268. https://doi.org/10.1002/(SICI)1099-1050(199905)8:3<263::AID-HEC426>3.0.CO;2-S
Miller A C (1975) The value of sequential information. Manag Sci 22(1):1–11. https://doi.org/10.1287/mnsc.22.1.1
Pozzi M, Der Kiureghian A (2011) Assessing the value of information for long-term structural health monitoring. In: Kundu T (ed) Proceedings of SPIE 7984, Health Monitoring of Structural and Biological Systems 2011. https://doi.org/10.1117/12.881918, p 79842W
Eckermann S, Willan A R (2008) Time and expected value of sample information wait for no patient. Value Health 11(3):522–526. https://doi.org/10.1111/j.1524-4733.2007.00296.x,
Memarzadeh M, Pozzi M (2016) Value of information in sequential decision making: component inspection, permanent monitoring and system-level scheduling. Reliab Eng Syst Saf 154:137–151. https://doi.org/10.1016/j.ress.2016.05.014
Dong H, Coyle D, Buxton M (2007) Value of information analysis for a new technology: computer-assisted total knee replacement. Int J Technol Assess Health Care 3(3):337–342. https://doi.org/10.1017/S0266462307070419
Martikainen J A, Kivioja A, Hallinen T, Vihinen P (2005) Economic evaluation of temozolomide in the treatment of recurrent glioblastoma multiforme. PharmacoEconomics 23(8):803–815. https://doi.org/10.2165/00019053-200523080-00006
Ginnelly L, Claxton K, Sculpher M J, Golder S (2005) Using value of information analysis to inform publicly funded research priorities. Appl Health Econ Health Policy 4(1):37–46. https://doi.org/10.2165/00148365-200504010-00006
Long E F, Vaidya N K, Brandeau M L (2008) Controlling co-epidemics: analysis of HIV and tuberculosis infection dynamics. Oper Res 56(6):1366–1381. https://doi.org/10.1287/opre.1080.0571
Lee C P, Chertow G M, Zenios S A (2008) Optimal initiation and management of dialysis therapy. Oper Res 56(6):1428–1449. https://doi.org/10.1287/opre.1080.0613
Chen Q, Ayer T, Chhatwal J (2018) Optimal M-switch surveillance policies for liver cancer in a hepatitis C-infected population. Oper Res 66(3):673–696. https://doi.org/10.1287/opre.2017.1706
Chan T, Narasimhan C, Xie Y (2013) Treatment effectiveness and side effects: a model of physician learning. Manag Sci 59(6):1309–1325. https://doi.org/10.1287/mnsc.1120.1640
Long E F, Nohdurft E, Spinler S (2018) Spatial resource allocation for emerging epidemics: a comparison of greedy, myopic, and dynamic policies. Manuf Serv Oper Manag 20(2):181–198. https://doi.org/10.1287/msom.2017.0681
Liu S, Brandeau M L, Goldhaber-Fiebert J D (2017) Optimizing patient treatment decisions in an era of rapid technological advances: the case of hepatitis C treatment. Health Care Manag Sci 20(1):16–32. https://doi.org/10.1007/s10729-015-9330-6
Negoescu D M, Bimpikis K, Brandeau M L, Iancu D A (2017) Dynamic learning of patient response types: an application to treating chronic diseases. Manag Sci. https://doi.org/10.1287/mnsc.2017.2793
Denton B T, Alagoz O, Holder A, Lee E K (2011) Medical decision making: open research challenges
Capan M, Khojandi A, Denton B T, Williams K D, Ayer T, Chhatwal J, Kurt M, Lobo J M, Roberts M S, Zaric G, Zhang S, Schwartz J S (2017) From data to improved decisions: operations research in healthcare delivery. Med Dec Making 37(8):849–859. https://doi.org/10.1177/0272989X17705636
Saville C E, Smith H K, Bijak K (2018) Operational research techniques applied throughout cancer care services: a review. Health Syst 6965:1–22. https://doi.org/10.1080/20476965.2017.1414741
Cooper K, Brailsford S C, Davies R, Raftery J (2006) A review of health care models for coronary heart disease interventions. Health Care Manag Sci 9(4):311–324. https://doi.org/10.1007/s10729-006-9996-x
Stanford R.E. (2004) A frontier analysis approach for benchmarking hospital performance in the treatment of acute myocardial infarction. Health Care Manag Sci 7:145–154. https://doi.org/10.1023/B:HCMS.0000020654.69499.50
Zargoush M, Gümü M, Verter V, Daskalopoulou S S (2018) Designing risk-adjusted therapy for patients with hypertension. Prod Oper Manag 27(12):2291–2312. https://doi.org/10.1111/poms.12872
Hauskrecht M, Fraser H (2000) Planning treatment of ischemic heart disease with partially observable Markov decision processes. Artif Intell Med 18:221–244. https://doi.org/10.1016/S0933-3657(99)00042-1
Denton B T, Kurt M, Shah N D, Bryant S C, Smith S (2009) Optimizing the start time of statin therapy for patients with diabetes. Med Dec Making 29(3):351–367. https://doi.org/10.1177/0272989X08329462
Kurt M, Denton B T, Schaefer A J, Shah N D, Smith S (2011) The structure of optimal statin initiation policies for patients with Type 2 diabetes. IIE Trans Healthcare Syst Eng 1:49–65. https://doi.org/10.1080/19488300.2010.550180
Mason J E, Denton B T, Shah N D, Smith S A (2014) Optimizing the simultaneous management of blood pressure and cholesterol for Type 2 diabetes patients. Eur J Oper Res 233(3):727–738. https://doi.org/10.1016/j.ejor.2013.09.018
Schell G J, Marrero W J, Lavieri M S, Sussman J B, Hayward R A (2016) Data-driven Markov decision process approximations for personalized hypertension treatment planning. MDM Policy Pract 1(1). https://doi.org/10.1177/2381468316674214
Hicklin K, Ivy J S, Payton F C, Viswanathan M, Myerse E (2018) Exploring the value of waiting during labor. Serv Sci 10(3):334–353. https://doi.org/10.1017/S002205070001648X
Chhatwal J, Alagoz O, Burnside E S (2010) Optimal breast biopsy decision-making based on mammographic features and demographic factors
Suen S-, Brandeau M L, Goldhaber-Fiebert J D (2018) Optimal timing of drug sensitivity testing for patients on first-line tuberculosis treatment. Health Care Manag Sci 21(4):632–646. https://doi.org/10.1007/s10729-017-9416-4
Agnihothri S, Cui L, Delasay M, Rajan B (2018) The value of mHealth for managing chronic conditions. Health Care Management Science. https://doi.org/10.1007/s10729-018-9458-2
Onen Z, Sayin S, Gurvit (2018) Optimal population screening policies for Alzheimer’s disease. IISE Trans Healthcare Syst Eng 5579:1–36. https://doi.org/10.1080/24725579.2018.1543738
Ayer T, Alagoz O, Stout N K, Burnside E S (2016) Heterogeneity in women’s adherence and its role in optimal breast cancer screening policies. Manag Sci 62(5):1339–1362. https://doi.org/10.1287/mnsc.2015.2180
Lee E, Lavieri M S, Volk M (2018) Optimal screening for hepatocellular carcinoma: a restless bandit model. Manuf Serv Oper Manag. https://doi.org/10.1287/msom.2017.0697
Deo S, Rajaram K, Rath S, Karmarkar U S, Goetz M B (2015) Planning for HIV screening, testing, and care at the veterans health administration. Oper Res 63(2):287–304. https://doi.org/10.1287/opre.2015.1353
Helm J E, Lavieri M S, Van Oyen M P, Stein J D, Musch D C (2015) Dynamic forecasting and control algorithms of glaucoma progression for clinician decision support. Oper Res 63(5):979–999. https://doi.org/10.1287/opre.2015.1405
Maillart L M, Ivy J S, Ransom S, Diehl K (2008) Assessing dynamic breast cancer screening policies. Oper Res 56(6):1411–1427. https://doi.org/10.1287/opre.1080.0614
Zhang J, Denton B T, Balasubramanian H, Shah N D, Inman B A (2012) Optimization of Prostate Biopsy Referral Decisions. Manuf Serv Oper Manag 14(4):529–547. https://doi.org/10.1287/msom.1120.0388
Erenay F S, Alagoz O, Said A (2014) Optimizing colonoscopy screening for colorectal cancer prevention and surveillance. Manuf Servi Oper Manag 16(3):381–400. https://doi.org/10.1287/msom.2014.0484
Skandari M R, Shechter S M, Zalunardo N (2015) Optimal vascular access choice for patients on hemodialysis. Manuf Serv Oper Manag 17(4):608–619. https://doi.org/10.1287/msom.2015.0552
Sabouri A, Huh W T, Shechter S M (2017) Screening strategies for patients on the kidney transplant waiting list. Oper Res 65(5):1131–1146. https://doi.org/10.1287/opre.2017.1632
Lin Y, Huang S, Simon G E, Liu S (2018) Data-based decision rules to personalize depression follow-up. Sci Rep 8(1):4–11. https://doi.org/10.1038/s41598-018-23326-1
Hutton D W, Tan D, So S K, Brandeau M L (2007) Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med 147(7):460. https://doi.org/10.7326/0003-4819-147-7-200710020-00004
Hassmiller Lich K, Cornejo D A, Mayorga M E, Pignone M, Tangka F KL, Richardson L C, Kuo T-M, Meyer A-M, Hall I J, Smith J L, Durham T A, Chall S A, Crutchfield T M, Wheeler S B (2017) Cost-effectiveness analysis of four simulated colorectal cancer screening interventions, North Carolina. Prevent Chron Disease 14(1):160158. https://doi.org/10.5888/pcd14.160158
Leshno M, Halpern Z, Arber N (2003) Cost-effectiveness of colorectal cancer screening in the average risk population. Health Care Manag Sci 6(3):165–174. https://doi.org/10.1023/A:1024488007043
Chirikos T N (2003) Appraising the economic efficiency of cancer treatment: an exploratory analysis of lung cancer. Health Care Manag Sci 6(2):87–95. https://doi.org/10.1023/A:1023380918605
Lin Y, Huang S, Simon G E, Liu S (2019) Cost-effectiveness analysis of prognostic-based depression monitoring. IISE Trans Healthcare Syst Eng 9(1):41–54. https://doi.org/10.1080/24725579.2019.1567627
Robins J, Orellana L, Rotnitzky A (2008) Estimation and extrapolation of optimal treatment and testing strategies. Stat Med 27(23):4678–4721. https://doi.org/10.1002/sim.3301
Kirkizlar E, Faissol D M, Griffin P M, Swann J L (2010) Timing of testing and treatment for asymptomatic diseases. Math Biosci 226(1):28–37. https://doi.org/10.1016/j.mbs.2010.03.007
Kazemian P, Helm J E, Lavieri M S, Stein J D, Van Oyen M P (2018) Dynamic monitoring and control of irreversible chronic diseases with application to glaucoma. Prod Oper Manag 0(0):poms.12975. https://doi.org/10.1111/poms.12975
Yang Y, Goldhaber-Fiebert J D, Wein L M (2013) Analyzing screening policies for childhood obesity. Manag Sci 59(4):782–795. https://doi.org/10.1287/mnsc.1120.1587, NIHMS150003
Ghamat S, Zaric G S, Pun H (2017) Contracts to promote optimal use of optional diagnostic tests in cancer treatment. Prod Oper Manag 27(12):2184–2200. https://doi.org/10.1111/poms.12780
Harper P R, Jones S K (2005) Mathematical models for the early detection and treatment of colorectal cancer. Health Care Manag Sci 8(2):101–109. https://doi.org/10.1007/s10729-005-0393-7
Brønnnum-Hansen H, Jørgensen T, Davidsen M, Madsen M, Osler M, Gerdes L U, Schroll M (2001) Survival and cause of death after myocardial infarction: the Danish MONICA study. J Clin Epidemiol 54(12):1244–1250. https://doi.org/10.1016/S0895-4356(01)00405-X
Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C (1994) Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire Community Stroke Project. Stroke 25 (2):333–7. https://doi.org/10.1161/01.STR.25.2.333
Khera A V, Chaffin M, Aragam K G, Haas M E, Roselli C, Choi S H, Natarajan P, Lander E S, Lubitz S A, Ellinor P T, Kathiresan S (2018) Genome-wide polygenic score to identify a monogenic risk-equivalent for coronary disease. Nat Genet 50(9):1219–1224. https://doi.org/10.1101/218388
Gold M R, Stevenson D, Fryback D G (2002) HALYs and QALYs and DALYs, Oh My: Similarities and Differences in Summary Measures of Population Health. Annu Rev Public Health 23(1):115–134. https://doi.org/10.1146/annurev.publhealth.23.100901.140513
Kleinbaum D G, Klein M (2005) Survival analysis: a self-learning text, 2nd edn. Springer, New York
Hoel M, Iversen T, Nilssen T, Vislie J (2006) Genetic testing in competitive insurance markets with repulsion from chance: A welfare analysis. J Health Econ 25(5):847–860. https://doi.org/10.1016/j.jhealeco.2005.12.003
Centers for Disease Control and Prevention (2017) National Health and Nutrition Examination Survey Data. https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx
Stekhoven D J, Buhlmann P (2012) MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics 28(1):112–118. https://doi.org/10.1093/bioinformatics/btr597
Nowok B, Raab G M, Dibben C (2016) synthpop: bespoke creation of synthetic data in R. J Stat Softw 74(11). https://doi.org/10.18637/jss.v074.i11, https://www.jstatsoft.org/article/view/v074i11
National Center for Health Statistics (2017) Health, United States, 2016: with chartbook on long-term trends in health https://www.ncbi.nlm.nih.gov/books/NBK453378/
Arias E, Xu J (2019) United States Life Tables, 2017. National Vital Statistics Reports 68(7)
Fryback D G, Dasbach E J, Klein R, Klein B E, Dorn N, Peterson K, Martin P A (1993) The beaver dam health outcomes study: initial catalog of health-state quality factors. Med Dec Making 13 (2):89–102
Pignone M, Earnshaw S, Tice J A, Pletcher M J (2006) Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: a cost-utility analysis. Ann Intern Med 144:326–336. https://doi.org/10.7326/0003-4819-144-5-200603070-00007
Pignone M (2007) Aspirin for the primary prevention of cardiovascular disease in women. Arch Intern Med 167(3):290. https://doi.org/10.1001/archinte.167.3.290
Pandya A, Sy S, Cho S, Weinstein M C, Gaziano T A (2015) Supplementary Online Content: Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. J Am Med Assoc 314(2):142–150. https://doi.org/10.1001/jama.2015.6822
O’Sullivan A K, Rubin J, Nyambose J, Kuznik A, Cohen D J, Thompson D (2011) Cost estimation of cardiovascular disease events in the US. PharmacoEconomics 29(8):693–704. https://doi.org/10.2165/11584620-000000000-00000
Medical Expenditure Panel Survey (2015) MEPS HC-163: 2013 full year consolidated data file. https://meps.ahrq.gov/data_stats/download_data/pufs/h163/h163doc.shtml
GoodRx (2017) Prescription prices, coupons & pharmacy information. https://www.goodrx.com/
Color Genomics (2018) Genetic testing for inherited heart conditions. https://www.color.com/product/hereditary-heart-health-genetic-test
Neumann PJ, Sanders GD, Russell LB, Siegel JE (2016) Cost-effectiveness in health and medicine. Oxford University Press
Abraham G, Havulinna A S, Bhalala O G, Byars S G, De Livera A M, Yetukuri L, Tikkanen E, Perola M, Schunkert H, Sijbrands E J, Palotie A, Samani N J, Salomaa V, Ripatti S, Inouye M (2016) Genomic prediction of coronary heart disease. Eur Heart J 37(43):3267–3278. https://doi.org/10.1093/eurheartj/ehw450
Kochanek K D, Murphy S L, Xu J, Arias E (2019) Deaths: final data for 2017. Natl Vital Stat Rep 68(9):1–18
Sussman J, Vijan S, Hayward R (2013) Using Benefit-Based Tailored Treatment to Improve the Use of Antihypertensive Medications. Circulation 128(21):2309–2317. https://doi.org/10.1161/CIRCULATIONAHA.113.002290
Pletcher M J, Pignone M, Earnshaw S, McDade C, Phillips K A, Auer R, Zablotska L, Greenland P (2014) Using the coronary artery calcium score to guide statin therapy. Circul Cardiovascul Qual Outcomes 7 (2):276–284. https://doi.org/10.1161/CIRCOUTCOMES.113.000799 NIHMS150003
Hayward R A, Krumholz H M, Zulman D M, Timbie J W, Vijan S (2010) Optimizing statin treatment for primary prevention of coronary artery disease. Ann Intern Med 152(2):69. https://doi.org/10.7326/0003-4819-152-2-201001190-00004
Ibrahim J G, Chen M-H, Sinha D (2001) Bayesian survival analysis. Springer Series in Statistics. Springer, New York. https://doi.org/10.1007/978-1-4757-3447-8
Ben-Tal A, Ghaoui L E, Nemirovski A (2009) Robust optimization. Princeton University Press
McNeil J J, Nelson M R, Woods R L, Lockery J E, Wolfe R, Reid C M, Kirpach B, Shah R C, Ives D G, Storey E, Ryan J, Tonkin A M, Newman A B, Williamson J D, Margolis K L, Ernst M E, Abhayaratna W P, Stocks N, Fitzgerald S M, Orchard S G, Trevaks R E, Beilin L J, Donnan G A, Gibbs P, Johnston C I, Radziszewska B, Grimm R, Murray A M (2018) Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med 379(16):1519–1528. https://doi.org/10.1056/nejmoa1803955
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement D L, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen S E, Kreutz R, Laurent S, Lip G Y H, McManus R, Narkiewicz K, Ruschitzka F, Schmieder R E, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, De Backer G, Heagerty A M, Agewall S, Bochud M, Borghi C, Boutouyrie P, Brguljan J, Bueno H, Caiani E G, Carlberg B, Chapman N, Cífková R, Cleland J G F, Collet J-P, Coman I M, de Leeuw P W, Delgado V, Dendale P, Diener H-C, Dorobantu M, Fagard R, Farsang C, Ferrini M, Graham I M, Grassi G, Haller H, Hobbs F D R, Jelakovic B, Jennings C, Katus H A, Kroon A A, Leclercq C, Lovic D, Lurbe E, Manolis A J, McDonagh T A, Messerli F, Muiesan M L, Nixdorff U, Olsen M H, Parati G, Perk J, Piepoli M F, Polonia J, Ponikowski P, Richter D J, Rimoldi S F, Roffi M, Sattar N, Seferovic P M, Simpson I A, Sousa-Uva M, Stanton A V, van de Borne P, Vardas P, Volpe M, Wassmann S, Windecker S, Zamorano J L, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman I M, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus H A, Knuuti J, Lancellotti P, Leclercq C, McDonagh T A, Piepoli M F, Ponikowski P, Richter D J, Roffi M, Shlyakhto E, Simpson I A, Sousa-Uva M, Zamorano J L, Tsioufis C, Lurbe E, Kreutz R, Bochud M, Rosei E A, Jelakovic B, Azizi M, Januszewics A, Kahan T, Polonia J, van de Borne P, Williams B, Borghi C, Mancia G, Parati G, Clement D L, Coca A, Manolis A, Lovic D, Benkhedda S, Zelveian P, Siostrzonek P, Najafov R, Pavlova O, De Pauw M, Dizdarevic-Hudic L, Raev D, Karpettas N, Linhart A, Olsen M H, Shaker A F, Viigimaa M, Metsärinne K, Vavlukis M, Halimi J-M, Pagava Z, Schunkert H, Thomopoulos C, Páll D, Andersen K, Shechter M, Mercuro G, Bajraktari G, Romanova T, Trušinskis K, Saade G A, Sakalyte G, Noppe S, DeMarco D C, Caraus A, Wittekoek J, Aksnes T A, Jankowski P, Polonia J, Vinereanu D, Baranova E I, Foscoli M, Dikic A D, Filipova S, Fras Z, Bertomeu-Martínez V, Carlberg B, Burkard T, Sdiri W, Aydogdu S, Sirenko Y, Brady A, Weber T, Lazareva I, Backer T D, Sokolovic S, Jelakovic B, Widimsky J, Viigimaa M, Pörsti I, Denolle T, Krämer B K, Stergiou G S, Parati G, Trušinskis K, Miglinas M, Gerdts E, Tykarski A, de Carvalho Rodrigues M, Dorobantu M, Chazova I, Lovic D, Filipova S, Brguljan J, Segura J, Gottsäter A, Pechère-Bertschi A, Erdine S, Sirenko Y, Brady A (2018) 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 39(33):3021–3104. https://doi.org/10.1093/eurheartj/ehy339
James P A, Oparil S, Carter B L, Cushman W C, Dennison-Himmelfarb C, Handler J, Lackland D T, LeFevre M L, MacKenzie T D, Ogedegbe O, Smith S C, Svetkey L P, Taler S J, Townsend R R, Wright J T, Narva A S, Ortiz E (2014) Evidence-based guideline for the management of high blood pressure in adults. J Am Med Assoc 311(5):507–20. https://doi.org/10.1001/jama.2013.284427
Kazi D S, Moran A E, Coxson P G, Penko J, Ollendorf D A, Pearson S D, Tice J A, Guzman D, Bibbins-Domingo K (2016) Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. J Am Med Assoc 316(7):743–. https://doi.org/10.1001/jama.2016.11004
United States Department of Health and Human Services (2006) Coverage and Reimbursement of Genetic Tests and Services: Report of the Secretary’s Advisory Committee on Genetics, Health, and Society, pp 1–57
Funding
Wesley J. Marrero’s work on this study was supported in part by the National Science Foundation grant DGE 1256260. Mariel S. Lavieri’s work was supported in part by the National Science Foundation grant CMMI-1552545. Jeremy B. Sussman’s work was supported in part by the United States Department of Veterans Affairs grants VA HSR&D CDA13-021 and VA HSR&D IIR 15-432. The National Science Foundation and the Veterans Affairs had no influence on the study’s design, conduct, and/or reporting.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Wesley J. Marrero. The first draft of the manuscript was written by Wesley J. Marrero and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethics Approval
In accordance with the policies of the University of Michigan, ethics approval was not needed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix
1.1 A.1 Progression of Risk Factors Over Time
A.2 Convergence Analysis
A.3 Results of Sensitivity Analysis by Age Groups
Rights and permissions
About this article
Cite this article
Marrero, W.J., Lavieri, M.S. & Sussman, J.B. Optimal cholesterol treatment plans and genetic testing strategies for cardiovascular diseases. Health Care Manag Sci 24, 1–25 (2021). https://doi.org/10.1007/s10729-020-09537-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10729-020-09537-x