Abstract
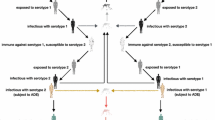
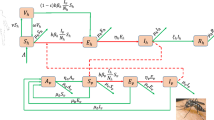
Dengue fever is a vector-borne disease prevalent in tropical and subtropical regions. It is an important public health problem with a considerable and often under-valued disease burden in terms of frequency, cost and quality-of-life. Recent literature reviews have documented the development of mathematical models of dengue fever both to identify important characteristics for future model development as well as to assess the impact of dengue control interventions. Such reviews highlight the importance of short-term cross-protection; antibody-dependent enhancement; and seasonality (in terms of both favourable and unfavourable conditions for mosquitoes). The compartmental model extends work by Bartley (2002) and combines the following factors: seasonality, age-structure, consecutive infection by all four serotypes, cross-protection and immune enhancement, as well as combined vector-host transmission. The model is used to represent dengue transmission dynamics using parameters appropriate for Thailand and to assess the potential impact of combined vector-control and vaccination strategies including routine and catch-up vaccination strategies on disease dynamics. When seasonality and temporary cross-protection between serotypes are included, the model is able to approximate the observed incidence of dengue fever in Thailand. We find vaccination to be the most effective single intervention, albeit with imperfect efficacy (30.2 %) and limited duration of protection. However, in combination, control interventions and vaccination exhibit a marked impact on dengue fever transmission. This study shows that an imperfect vaccine can be a useful weapon in reducing disease spread within the community, although it will be most effective when promoted as one of several strategies for combating dengue fever transmission.


Similar content being viewed by others
References
WHO (2011) Working to overcome the global impact of neglected tropical diseases Update 2011. WHO/HTM/NTD/2011.3 (World Health Organization)
Gubler DJ (1998) Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 11(3):480–496
Gubler DJ (2002) Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol 10(2):100–103
WHO (2009) Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. WHO/HTM/NTD/DEN/2009.1 (World Health Organization)
Wearing HJ, Rohani P (2006) Ecological and immunological determinants of dengue epidemics. Proc Natl Acad Sci U S A 103(31):11802–11807
Halstead SB (2004) Antibody-dependent enhancement of infection: a mechanism for indirect virus entry into cells. Cellular Receptor for Animal Viruses, Cold Spring Harbor Laboratory Press 28:493–516. 0-87969-429-7/94 (Chapter 25)
WHO (2007) Report of the Scientific Working Group meeting on dengue. (World Health Organization). Available: http://apps.who.int/tdr/svc/publications/tdr-research-publications/swgreport-dengue. Accessed March 20, 2013.
Halstead SB (2007) Dengue Lancet 370(9599):1644–1652
Aguiar M, Stollenwerk N, Kooi BW (2009) Torus bifurcations, isolas and chaotic attractors in a simple dengue model with ADE and temporary cross immunity (Chapter 3). Int J Comput Math 86:1867–1877
Aguiar M et al (2011) The role of seasonality and import in a minimalistic multi-strain dengue model capturing differences between primary and secondary infections: complex dynamics and its implications for data analysis (Chapter 4). J Theor Biol 289:181–196
WHO (2012) Dengue and severe dengue - Fact sheet N°117, http://www.who.int/mediacentre/factsheets/fs117/en/ (Accessed 2012-03-27)
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI (2013) The global distribution and burden of dengue. Nature 496(7446):504–507
Gubler DJ, Wilson ML (2005) The global resurgence of vector-borne diseases: lessons learned from successful and failed adaptation, Integration of public health with adaptation to climate change: lessons learned and new directions. In: Ebi KL, Smith J, Burton I (eds) Taylor and Francis, London, pp 44–59
Asia-Pacific Dengue Program Managers Meeting [proceedings] (2008) World Health Organization Western Pacific Region: National Environment Agency
Kongnuy R, Pongsumpun P (2011) Mathematical Modeling for Dengue Transmission with the Effect of Season. World Academy of Science, Engineering and Technology 51
Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, Scott TW (2011) Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. PNAS 108(18):7460–7465
Massad E, Coutinho FA (2011) The cost of dengue control. Lancet 377(9778):1630
Guzmán MG, Kouri G (2002) Dengue: an update. Lancet Infect Dis 2(1):33–42
Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J (2012) Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 380(9853):1559–1567
WHO (2011) Regional Office for South-East Asia. Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Haemorrhagic Fever; Revised and expanded edition. (SEARO Technical Publication Series No. 60)
Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R (2008) Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med 18:5(3)
Derouich M, Boutayeb A, Twizell EH (2003) A model of dengue fever. Biomed Eng Online 2:4
Derouich M, Boutayeb A (2006) Dengue fever: Mathematical modelling and computer simulation. Appl Math Comput 177:528–544
Bartley LM, Donnelly CA, Garnett GP (2002) The seasonal pattern of dengue in endemic areas: mathematical models of mechanisms. Trans R Soc Trop Med Hyg 96(4):387–397
Focks DA, Daniels E, Haile DG, Keesling JE (1995) A simulation model of the epidemiology of urban dengue fever: Literature analysis, model development, preliminary validation and samples of simulation results. Am J Trop Med Hyg 53(5):489–506
Nagao Y, Koelle K (2008) Decreases in dengue transmission may act to increase the incidence of dengue hemorrhagic fever. Proc Natl Acad Sci U S A 105(6):2238–2243
Esteva L, Vargas C (1998) Analysis of a dengue disease transmission model. Math Biosci 150(2):131–151
Chikaki E, Ishikawa H (2009) A dengue transmission model in Thailand considering sequential infections with all four serotypes. J Infect Dev Ctries 3(9):711–722
Otero M, Barmak DH, Dorso CO, Solari HG, Natiello MA (2011) Modeling dengue outbreaks. Math Biosci 232(2):87–95
Newton EAC, Reiter P (1992) A model of the transmission of dengue fever with an evaluation of the impact of ultralow volume (ULV) insecticide applications of dengue epidemics. Am J Trop Med Hyg 47:709–720
Burattini MN, Chen M, Chow A, Coutinho FA, Goh KT, Lopez LF, Ma S, Massad E (2008) Modelling the control strategies against dengue in Singapore. Epidemiol Infect 136(3):309–319
Derouich M (2009) Dengue Fever: A Mathematical Model with Immunization Program in Handbook of Research on Systems Biology Applications in Medicine. Editor Andriani Daskalaki (Max Planck Institute for Molecular Genetics, Germany)
Luz PM, Codeco CT, Medlock J, Struchiner CJ, Valle D et al (2009) Impact of insecticide interventions on the abundance and resistance profile of Aedes aegypti. Epidemiol Infect 137(8):1203–1215
Yang HM, Ferreira CP (2008) Assessing the effects of vector control on dengue transmission. Appl Math Comput 198:401–413
Rodrigues HS, Monteiro MTT, Torres DFM, Zinober A (2010a) Control of Dengue disease: a case study in Cape Verde. Proc. 10th International Conference on Mathematical Methods in Science and Engineering, Almerıa, pp 26–30
Rodrigues HS, Monteiro MTT, Torres DFM (2010b) Insecticide control in a Dengue epidemics model. In: Simos T (ed) Numerical Analysis and Applied Mathematics. AIP Conf. Proc, 1281 pp 979–982
Oki M, Sunahara T, Hashizume M, Yamamoto T (2011) Optimal timing of insecticide fogging to minimize dengue cases: modeling dengue transmission among various seasonalities and transmission intensities. PLoS Negl Trop Dis 5(10):e1367
Coudeville L, Garnett GP (2012) Transmission dynamics of the four dengue serotypes in southern Vietnam and the potential impact of vaccination. PLoS One 7(12):e51244
Amaku M, Coutinho FAM, Raimundo SM, Lopez LF, Burrattini MN, Massad E. A Comparative Analysis of the Relative Efficacy of Vector-Control Strategies against Dengue Fever. Manuscript under review. http://arxiv.org/abs/1302.1166.
Chao DL, Halstead SB, Halloran ME, Longini IM Jr (2012) Controlling dengue with vaccines in Thailand. PLoS Negl Trop Dis 6(10):e1876
Barmak DH, Dorso CO, Otero M, Solari HG (2013) Modelling interventions during a dengue outbreak. Epidemiol Infect 26:1–17
Boccia TM, Burattini MN, Coutinho FA, Massad E (2013) Will people change their vector-control practices in the presence of an imperfect dengue vaccine? Epidemiol Infect 5:1–9
Nishiura H (2006) Mathematical and statistical analyses of the spread of dengue. Dengue Bull 30:51–67, Table 1, p53
Johansson MA, Hombach J, Cummings DAT (2011) Models of the impact of dengue vaccines: A review of current research and potential approaches. Vaccine 29(35):5860–5868
Andraud M, Hens N, Marais C, Beutels P (2012) Dynamic epidemiological models for dengue transmission: a systematic review of structural approaches. PLoS One 7(11):e49085
Bureau of Epidemiology (2008) Ministry of Public Health, Thailand
Wichmann O, Yoon IK, Vong S, Limkittikul K, Gibbons RV, Mammen MP, Ly S, Buchy P, Sirivichayakul C, Buathong R, Huy R, Letson GW, Sabchareon A (2011) Dengue in Thailand and Cambodia: an assessment of the degree of underrecognized disease burden based on reported cases. PLoS Negl Trop Dis 5(3):e996
Undurraga EA, Halasa YA, Shepard DS (2013) Use of expansion factors to estimate the burden of dengue in Southeast Asia: a systematic analysis. PLoS Negl Trop Dis 7(2):e2056
Luz PM, Vanni T, Medlock J, Paltiel AD, Galvani AP (2011) Dengue vector control strategies in an urban setting: an economic modelling assessment. Lancet 377(9778):1673–1680
Anderson RM, May RM (1991) Infectious Diseases of Humans, Dynamics and Control. Oxford University Press, Oxford
Coutinho FAB, Burattini MN, Lopez LF, Massad E (2005) An approximate threshold condition for non-autonomous system: An application to a vector-borne infection. Math Comput Simul 70:149–158
Coutinho FAB, Burattini MN, Lopez LF, Massad E (2006) Threshold conditions for a nonautonomous epidemic system describing the population dynamics of dengue. Bull Math Biol 68(8):2263–2282
Berkeley Madonna 8.02 (2009)
Gubler DJ, Kuno G (eds) (2001) Dengue and dengue hemorrhagic fever. CABI Publishing, Wallingford, UK 425–62
Chang MS et al (2011) Challenges and future perspective for dengue vector control in the Western Pacific Region. W Pac Surveill Response J 2(2):9–16
McCall PJ, Kittayapong P (2006) Control of dengue vectors: tools and strategies. Working paper for the Scientific Working Group on Dengue Research, convened by the Special Programme for Research and Training in Tropical Diseases, Geneva, 1–5
Nathan MB, Focks DA, Kroeger A (2006) Pupal/demographic surveys to inform dengue-vector control. Ann Trop Med Parasitol 100(Suppl 1):S1–S3
Focks D, Alexander N. Multicountry study of Aedes aegypti pupal productivity survey methodology: findings and recommendations. TDR/IRM/Den /06.1 Copyright © World Health Organization on behalf of the Special Programme for Research and Training in Tropical Diseases, 2006.
Tun-Lin W, Lenhart A, Nam VS, Rebollar-Téllez E, Morrison AC, Barbazan P, Cote M, Midega J, Sanchez F, Manrique-Saide P, Kroeger A, Nathan MB, Meheus F, Petzold M (2009) Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: a multi-country non-inferiority cluster randomized trial. Trop Med Int Health 14(9):1143–1153
WHO HELI (2013) Better environmental management for control of dengue. Health and Environment Linkages Initiative (HELI) - Accessed 15 June/2013: http://www.who.int/heli/risks/vectors/denguecontrol/en/
Marcombe S, Mathieu RB, Pocquet N, Riaz MA, Poupardin R, Sélior S, Darriet F, Reynaud S, Yébakima A, Corbel V, David JP, Chandre F (2012) Insecticide resistance in the dengue vector Aedes aegypti from Martinique: distribution, mechanisms and relations with environmental factors. PLoS One 7(2):e30989
Ranson H, Joseph Burhani J, Nongkran, Lumjuan N, Black WC (2010) Insecticide resistance in dengue vectors. TropIKA.net vol.1 no.1 Jan./Mar. 2010
Yellow fever Fact sheet N°100 (2013). Accessed 30 June 2013: http://www.who.int/mediacentre/factsheets/fs100/en/
Nam VS, Thi Yen N, Minh Duc H, Cong Tu T, Trong Thang V, Le Hoang N, Hoang San L, Le Loan L, Que Huong VT, Kim Khanh LH, Thuy Trang HT, Lam LZ, Kutcher SC, Aaskov JG, Jeffery JA, Ryan PA, Kay BH (2012) Community-based control of Aedes aegypti by using Mesocyclops in southern Vietnam. Am J Trop Med Hyg 86(5):850–859
Vu SN, Nguyen TY, Kay BH, Marten GG, Reid JW (1998) Eradication of Aedes aegypti from a village in Vietnam, using copepods and community participation. Am J Trop Med Hyg 59(4):657–660
Vu SN, Nguyen TY, Tran VP, Truong UN, Le QM, Le VL, Le TN, Bektas A, Briscombe A, Aaskov JG, Ryan PA, Kay BH (2005) Elimination of dengue by community programs using Mesocyclops(Copepoda) against Aedes aegypti in central Vietnam. Am J Trop Med Hyg 72(1):67–73
Cattand P, Desjeux P, Guzmán MG, Jannin J, Kroeger A, Médici A, Musgrove P, Nathan MB, Shaw A, Schofield CJ (2006) Tropical Diseases Lacking Adequate Control Measures: Dengue, Leishmaniasis, and African Trypanosomiasis. Disease Control Priorities in Developing Countries, 2nd edn. Oxford University Press, New York, pp 451–466. doi:10.1596/978-0-821-36179-5/Chpt-23
Lloyd AL, Zhang J, Root AM (2007) Stochasticity and heterogeneity in host-vector models. J R Soc Interface 4(16):851–863
Woolhouse ME, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, Hagan P, Hii JL, Ndhlovu PD, Quinnell RJ, Watts CH, Chandiwana SK, Anderson RM (1997) Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci U S A 94(1):338–342
De Benedictis J, Chow-Shaffer E, Costero A, Clark GG, Edman JD, Scott TW (2003) Identification of the people from whom engorged Aedes aegypti took blood meals in Florida, Puerto Rico, using polymerase chain reaction-based DNA profiling. Am J Trop Med Hyg 68(4):437–446
Rahmandad H, Sterman J (2008) Heterogeneity and network structure in the dynamics of diffusion: Comparing agent-based and differential equation models. Manag Sci 54(5):998–1014
Jacintho LFO, Batista AFM, Ruas TL, Marietto MGB, Silva FA (2010) An agent-based model for the spread of the Dengue fever: a swarm platform simulation approach. An agent-based model for the spread of the Dengue fever: a swarm platform simulation approach. SpringSim ‘10: Proceedings of the 2010 Spring Simulation Multiconference
Brandeau ML, Zaric GS, Richter A (2003) Resource-allocation for control of infectious diseases in multiple independent populations: beyond cost-effectiveness analysis. J Health Econ 22:575–598
For 1970–2000, Thailand, source: United Nations
Luz PM, Lima-Camara TN, Bruno RV, Castro MG, Sorgine MH, Lourenço-de-Oliveira R, Peixoto AA (2011) Potential impact of a presumed increase in the biting activity of dengue-virus-infected Aedes aegypti (Diptera: Culicidae) females on virus transmission dynamics. Mem Inst Oswaldo Cruz 106(6):755–758
Acknowledgments
The authors wish to thank Julie Roiz for her expert insights into infectious disease modelling and Eduardo Massad for his patient answering of queries related to his work in dengue modelling.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Knerer, G., Currie, C.S.M. & Brailsford, S.C. Impact of combined vector-control and vaccination strategies on transmission dynamics of dengue fever: a model-based analysis. Health Care Manag Sci 18, 205–217 (2015). https://doi.org/10.1007/s10729-013-9263-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10729-013-9263-x