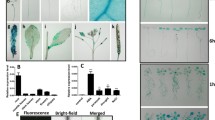
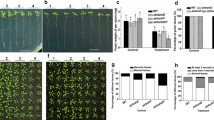
Abstract
Plants suffer from oxygen deficiency (hypoxia) and energy starvation under flooding conditions. Higher plants have evolved complex adaptive mechanisms to flooding that are induced by changes in the cellular redox state and phytohormones. Previously, we showed that the transcript levels of respiratory burst oxidase homolog I (AtRBOH I) in Arabidopsis increase under hypoxic stress. In this study, we used two independent Atrboh I-knockout lines to assess the molecular function of AtRBOH I in hypoxic signaling pathways. Under submergence conditions, the Atrboh I-knockout lines had a reduced survival rate and lower chlorophyll contents than those of wild type. The patterns of AtRBOH I expression were analyzed by fusing its promoter to the GUS reporter. These expression analyses indicated that AtRBOH I expression was activated by hypoxia, but this induction was reduced by the auxin transport inhibitor 1-naphthylphthalamic acid (NPA). Quantitative RT-PCR analyses showed that the transcript levels of hypoxia-inducible genes (AtHRE1, AtADH1, AtLDH, and AtSUS1) were reduced in AtRBOH I-knockout lines under hypoxic conditions. The transcript levels of AtSUS1 were lower in AtRBOH I-knockout lines than in wild type in the hypoxia combined with NPA treatment. Hypoxic conditions increased the transcript levels of the auxin-responsive genes At1g19840, At3g23030, and At5g19140, and hypoxia combined with NPA resulted in increased transcript levels of the ethylene biosynthetic genes AtACS7 and AtACS8. Together, these results show that AtRBOH I regulates the expression of genes involved in ethylene biosynthesis and down-stream of hypoxia signaling, and that there is some interplay between hypoxia signaling and auxin-mediated signaling pathways under hypoxic stress.





Similar content being viewed by others
References
Andres-Colas N, Perea-Garcia A, Mayo de Andres S, Garcia-Molina A, Dorcey E, Rodriguez-Navarro S, Perez-Amador MA, Puig S, Penarrubia L (2013) Comparison of global responses to mild deficiency and excess copper levels in Arabidopsis seedlings. Metallomics 5(9):1234–1246. doi:10.1039/c3mt00025g
Ashraf M, Arfan M (2005) Gas exchange characteristics and water relations in two cultivars of Hibiscus esculentus under waterlogging. Biol Plantarum 49(3):459–462. doi:10.1007/s10535-005-0029-2
Bailey-Serres J, Voesenek LA (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59:313–339. doi:10.1146/annurev.arplant.59.032607.092752
Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65(5):1229–1240. doi:10.1093/jxb/ert375
Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J (2002) RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296(5575):2026–2028. doi:10.1126/science.1071505
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Borisjuk L, Macherel D, Benamar A, Wobus U, Rolletschek H (2007) Low oxygen sensing and balancing in plant seeds: a role for nitric oxide. New Phytol 176(4):813–823. doi:10.1111/j.1469-8137.2007.02226.x
Cho HY, Wen TN, Wang YT, Shih MC (2016) Quantitative phosphoproteomics of protein kinase SnRK1 regulated protein phosphorylation in Arabidopsis under submergence. J Exp Bot 67(9):2745–2760. doi:10.1093/jxb/erw107
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36(8):665–681. doi:10.1071/FP09144
Finkel T (2000) Redox-dependent signal transduction. FEBS Lett 476(1–2):52–54
Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422(6930):442–446. doi:10.1038/nature01485
Fukao T, Xu K, Ronald PC, Bailey-Serres J (2006) A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 18(8):2021–2034. doi:10.1105/tpc.106.043000
Garrido I, Caraballo-Sanchez AM, Llerena JL, Espinosa F (2012) Oxidative reactions in axenically seedling roots of olive (Olea europaea, L.). Plant Growth Regul 68(2):203–210. doi:10.1007/s10725-012-9708-0
Gupta KJ, Igamberdiev AU (2016) Reactive nitrogen species in mitochondria and their implications in plant energy status and hypoxic stress tolerance. Front Plant Sci. doi:10.3389/Fpls.2016.00360
Huang SJ, Chang CL, Wang PH, Tsai MC, Hsu PH, Chang IF (2013) A type III ACC synthase, ACS7, is involved in root gravitropism in Arabidopsis thaliana. J Exp Bot 64(14):4343–4360. doi:10.1093/jxb/ert241
Iglesias MJ, Terrile MC, Bartoli CG, D’Ippolito S, Casalongue CA (2010) Auxin signaling participates in the adaptative response against oxidative stress and salinity by interacting with redox metabolism in Arabidopsis. Plant Mol Biol 74(3):215–222. doi:10.1007/s11103-010-9667-7
Jefferson RA (1989) The GUS reporter gene system. Nature 342(6251):837–838. doi:10.1038/342837a0
Licausi F, Perata P (2009) Low oxygen signaling and tolerance in plants. Adv Bot Res 50:139–198. doi:10.1016/S0065-2296(08)00804-5
Liu FL, VanToai T, Moy LP, Bock G, Linford LD, Quackenbush J (2005) Global transcription profiling reveals comprehensive insights into hypoxic response in Arabidopsis. Plant Physiol 137(3):1115–1129. doi:10.1104/pp.104.055475
Liu B, Rennenberg H, Kreuzwieser J (2015) Hypoxia induces stem and leaf nitric oxide (NO) emission from poplar seedlings. Planta 241(3):579–589. doi:10.1007/s00425-014-2198-8
Mach JM, Castillo AR, Hoogstraten R, Greenberg JT (2001) The Arabidopsis-accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc Natl Acad Sci USA 98(2):771–776. doi:10.1073/pnas.021465298
Mathur J (2004) Cell shape development in plants. Trends Plant Sci 9(12):583–590. doi:10.1016/j.tplants.2004.10.006
Muller K, Carstens AC, Linkies A, Torres MA, Leubner-Metzger G (2009) The NADPH-oxidase AtrbohB plays a role in Arabidopsis seed after-ripening. New Phytol 184(4):885–897. doi:10.1111/j.1469-8137.2009.03005.x
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5(5):388–395. doi:Doi:10.1016/S1369-5266(02)00282-0
Oda T, Hashimoto H, Kuwabara N, Akashi S, Hayashi K, Kojima C, Wong HL, Kawasaki T, Shimamoto K, Sato M, Shimizu T (2010) Structure of the N-terminal regulatory domain of a plant NADPH oxidase and its functional implications. J Biol Chem 285(2):1435–1445. doi:10.1074/jbc.M109.058909
Orman-Ligeza B, Parizot B, Rycke R, Fernandez A, Himschoot E, Breusegem F, Bennett MJ, Périlleux C, Beeckman T, Draye X (2016) RBOH-mediated ROS production facilitates lateral root emergence in Arabidopsis. Development 143:3328–3339. doi:10.1242/dev.136465
Peer WA, Cheng Y, Murphy AS (2013) Evidence of oxidative attenuation of auxin signalling. J Exp Bot 64(9):2629–2639. doi:10.1093/jxb/ert152
Remans T, Opdenakker K, Smeets K, Mathijsen D, Vangronsveld J, Cuypers A (2010) Metal-specific and NADPH oxidase dependent changes in lipoxygenase and NADPH oxidase gene expression in Arabidopsis thaliana exposed to cadmium or excess copper. Funct Plant Biol 37(6):532–544. doi:10.1071/FP09194
Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R (2004) Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 16(3):616–628. doi:10.1105/tpc.019398
Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14(6):691–699. doi:10.1016/j.pbi.2011.07.014
Tester M, Langridge P (2010) Breeding technologies to increase crop production in a changing world. Science 327(5967):818–822. doi:10.1126/science.1183700
Wu YS, Yang CY (2016) Physiological responses and expression profile of NADPH oxidase in rice (Oryza sativa) seedlings under different levels of submergence. Rice. doi:10.1186/S12284-016-0074-9
Xu T, Wen M, Nagawa S, Fu Y, Chen JG, Wu MJ, Perrot-Rechenmann C, Friml J, Jones AM, Yang Z (2010) Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 143(1):99–110. doi:10.1016/j.cell.2010.09.003
Xu QT, Yang L, Zhou ZQ, Mei FZ, Qu LH, Zhou GS (2013) Process of aerenchyma formation and reactive oxygen species induced by waterlogging in wheat seminal roots. Planta 238(5):969–982. doi:10.1007/s00425-013-1947-4
Yang CY (2014) Hydrogen peroxide controls transcriptional responses of ERF73/HRE1 and ADH1 via modulation of ethylene signaling during hypoxic stress. Planta 239(4):877–885. doi:10.1007/s00425-013-2020-z
Yang CY, Hong CP (2015) The NADPH oxidase Rboh D is involved in primary hypoxia signalling and modulates expression of hypoxia-inducible genes under hypoxic stress. Environ Exp Bot 115:63–72. doi:10.1016/j.envexpbot.2015.02.008
Acknowledgements
We thank Dr. Chang-Hsien Yang (Institute of Biotechnology, National Chung Hsing University, Taichung, Taiwan) for providing the pEpyon-01K vector for construction of GUS transgenic plants.
Author Contributions
The work presented here was carried out in collaboration between all authors. Dr. JTCT and Dr. C-YY collaboration defined the research theme. Dr. C-YY designed experiments, analyzed the data and wrote the manuscript. I-SL, Y-SW, C-TC and G-HC carried out the experiments and analyzed the data. G-YJ and S-GH help the Arabidopsis transformation and GUS analysis.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.

10725_2017_292_MOESM2_ESM.jpg
Sup. Fig. 1 Molecular analyses of rboh I-knockout lines. (A) Schematic of rbohd-ko-831 (salk_031831) and rbohd-ko-627 (salk_027627C) T-DNA insertion sites. (B) Quantitative RT-PCR analyses; mRNA levels of RBOH I in roots of 14-d-old wild type and two rboh I-knockout lines, rbohd-ko-831 and rbohd-ko-627, were determined by quantitative RT-PCR after 0 and 9 h of hypoxia treatment. Relative transcript levels were calculated and normalized to that of ACTIN mRNA. Values represent means ± standard deviation from three biologically independent experiments (n = 3). Asterisk indicates significant difference from wild type (P < 0.05; one-way ANOVA, Dunnett’s t-test) (JPG 75 KB)
Rights and permissions
About this article
Cite this article
Lin, IS., Wu, YS., Chen, CT. et al. AtRBOH I confers submergence tolerance and is involved in auxin-mediated signaling pathways under hypoxic stress. Plant Growth Regul 83, 277–285 (2017). https://doi.org/10.1007/s10725-017-0292-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-017-0292-1