Abstract
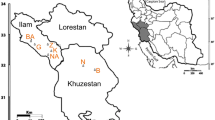
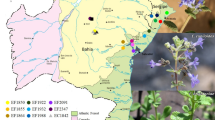
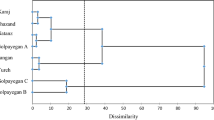
Orychophragmus violaceus, a ground covering plant that is widely distributed in China. It has both high economical value in food, forage, health care and ornamental value in gardening. In this study, the genetic diversity of 245 individuals from nine populations in China were investigated using the inter-simple sequence repeat markers. Of the 100 primers screened, eight were highly polymorphic. Using these primers, 162 discernible DNA fragments were generated with 150 (92.59%) being polymorphic, indicating a pronounced genetic variation at the species level. Also, there were high levels of polymorphism at the population level with the percentage of polymorphic bands ranging from 85.74 to 90.06%. Analysis of molecular variance showed that the genetic variation within populations was 80.80% and the variance among populations was 16.43%. The Nei’s GST (0.1643) and gene flow among populations (Nm = 2.5760) revealed large gene exchanges among populations. O. violaceus belongs to out-crossing plants. It is capable of reproducing by self-sowing, thus can influence population genetic structure. The pronounced genetic variation within populations tells us that O. violaceus is a proper plant for genetic research and that there is great potential of breeding this species for gardening.





Similar content being viewed by others
References
An XH, Chen BY, Fu TD, Liu HL (1999) Genetic diversity of Chinese landraces in Brassica napus was analysed by RAPD markers. J Huazhong Agric Univ 6:524–527
Balloux F, Lugon-Moulin B (2002) The estimation of population differentiation with microsatellite markers. Mol Ecol 11:155–165
Bawa KS, Ashton PS (1991) Conservation of rare trees in tropical rain forests: a genetic perspective. In: Falk DA, Holsinger KE (eds) Genetics and conservation of rare plants. Oxford University Press, New York, pp 62–67
Bolibok H, Pakoczy-Trojanowska M, Hronada A, Pietrzykowski R (2005) Efficiency of different PCR-based marker systems in assessing genetic diversity among winter rye (Secale cereale L.) inbred lines. Euphytica 146:109–116
Burton WA, Ripley VL, Potts DA, Salisbury PA (2004) Assessment of genetic diversity in selected breeding lines and cultivars of canola quality Brassica juncea and their implications for canola breeding. Euphytica 136:181–192
Bussell JD (1999) The distribution of random amplified polymorphic DNA (RAPD) diversity amongst populations of Isotoma petraea (Lobeliaceae). Mol Ecol 8:775–789
Camacho JC, Liston A (2001) Population structure and genetic diversity of Botrychium pumicola (Ophioglossaceae) based on inter-simple sequence repeats (ISSR). Am J Bot 81:965–972
Carvalho A, Matos M, Lima-Brito J, Guedes-Pinto H, Benito C (2005) DNA fingerprint of F1 interspecific hybrids from the triticeae tribe using ISSRs. Euphytica 143:93–99
Donnelly MJ, Twonson H (2000) Evidence for extensive genetic differentiation among populations of the malaria vector Anopheles arabiensis eastern Africa. Insect Mol Biol 9:357–367
Doyle JJ, Doyle JK (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bulletin 19:11–15
Esayas A, Endashaw B, Tomas B (2005) Inter-simple sequence repeat (ISSR) variation in forest coffee trees (Coffea arabica L.) populations from Ethiopia. Genetica 124:213–221
Excoffier L, Smousse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Gao L, Yang B (2006) Genetic diversity of wild Cymbidium goeringii (Orchidaceae) populations from Hubei based on ISSR analysis. Biodivers Sci 3:250–257
Ge S (2001) Application of DNA molecular marker in conservation biology. In: Zou YP, Ge S, Wang XD (eds) Molecular marker in systematic and evolutionary botany. Science Press, Beijing, pp 140–149
Ge XJ, Sun M (1999) Reproductive biology and genetic diversity of a cryptoviviparous mangrove Aegiceras corniculatum (Myrtinaceae) using allozyme and inter-simple sequence repeat (ISSR) analysis. Mol Ecol 8:2061–2069
Hamrick JL, Godt MJW (1989) Allozyme diversity in plant species. In Brown AHD, Clegg MT, Kahler AL, Weir BS (eds) Plant population genetics, breeding and germplasm resources. Sinauer Associaties, Sunderland, pp 43-63
Hamrick JL, Godt MJW (1996) Conservation genetics of endemic plant species. In: Avise JC, Hamrick JL (eds) Conservation genetics: case histories from nature. Chapman and Hall, New York, pp 281–304
He YT, Tu JX, Fu TD, Li DR, Chen BY (2002) Genetic diversity of germplasm resources of Brassica campestris L. in China by RAPD markers. Acta Agronom Ica Sinica 5:697–703
Hogbin PM, Peakall R (1999) Evaluation of the contribution of genetic research to the management of the endangered plant Zieria prostrate. Conserv Biol 13:514–522
Jin Y, Lu BR (2003) Sampling strategy of genetic diversity. Biodivers Sci 11:155–161
Kar PK, Vijayan K, Mohandas TP, Nair CV, Saratchandra B, Thangavelu K (2005) Genetic variability and genetica structure of wild and semi-domestic populations of Tasar silkworm (Antheraea mylitta) ecorace daba as revealed through ISSR markers. Genetica 125:173–183
Li J (1998) Analysis of POD in different Orychophragmus violaceus. J Southwest Agric Univ 20:223–225
Li HS, Chen GZ (2004) Genetic diversity of mangrove plant Sonneratia caseolaris in Hainan island based on ISSR analysis. Acta Ecologica Sinica 24:1656–1662
Li ZX, Cao XD, Liu DX, Liu J, Zhang ZS (1994) A study on the karyotype of some Chinese variants of Zhuge Cai, Orychophragmus violaceus. Acta Agronomica Sinica 20:595–600
Liang MS, Tao Z (1997) Separation of Orychophragmus violaceus gene promoters. Southwest China J Agric Sciences 3:1–5
Lu Z, Wang Y, Peng Y, Korpelainen H, Li C (2006) Genetic diversity of populus cathayana Rehd populations in southwestern China revealed by ISSR markers. Plant Sci 170:407–412
Luo P, Huang BQ, Yin JM, Chen ZL, Chen YH, Lan ZQ (1998) A new forage genetic resource Orychophragmus violaceus (L.) O. E. Schulz. Genet Resour Crop Evol 45:491–494
Luo P, Ye Q, Lan ZQ (2000) A study on floral biology of seedings in vitro in Orychophragmus violaceus: Induction of flowers in seedings of O. violaceus cultured in vitro. Plant Cell Tiss Organ Cult 63:73–75
McCue KA, Buckler ES, Holtsford TP (1996) A hierarrchical view of gentic structure in the rare annual plant Clarkia springvillensis. Conserv Biol 10:1425–1434
Mei DS, Li YC, Hu Q (2003) Investigation of male sterile lines intergeneric somatic hybrids of Brassica napus (+) Orychophragmus violaceus and B. napus (+) Sinapis arvensis. Chinese J Oil Crop Sci 1:72–75
Nagaoka T, Ogihara Y (1997) Applicability of inter-simple sequence repeat polymorphism in wheat for use as DNA markers in comparison to RFLP and RAPD markers. Theor Appl Genet 93:133–139
Neel MC, Ellstrand NC (2003) Conservation of genetic diversity in the endangered plant Eriogonum ovalifolium var. vineum (Polygonaceae). Conserv Genet 4:337–352
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA 70:3321–3323
Neigel JE (2002) Is F ST obsolete? Conserv Genet 3:167–173
Nybom H (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol 5:1143–1150
Ren QJ, Yu JP, Zhang GL (1998) The integrative utilization of Orychophragmus violaceus Resources. Chinese Wild Plant Resour 2:24–25
Rohlf FJ (2000) NTSYS-pc. Numerical taxonomy and multivariate analysis system, Version 2.1. Exeter Software, Setautket, New York
Shen JX, Fu TD, Yang GS (2004) Relationship between hybrid performance and genetic diversity based on SSR and ISSR in Brassica napus L. Scientia Agricultura Sinica 4:477–483
Slatkin M (1985) Gene flow in natural populations. Annu Rev Ecol Syst 16:393–430
Vijayan K, Srivatsava PP, Nair CV, Awasthi AK, Tikader A, Sreenivasa B, Urs SR (2006) Molecular characterization and markers associated with yield traits in mulberry using ISSR markers. Plant Breed 125:298–301
Wang JB (2002) ISSR markers and their applications in plant genetics. Hereditas 24:613–616
Wang S, Miao X, Zhao W, Huang B, Fan M, Li Z, Huang Y (2005) Genetic diversity and population structure among strains of the entomopathogenic fungus, Bieauveria bassiana, as revealed by inter-simple sequence repeats (ISSR). Mycol Res 109:1364–1372
Weng DB, Huang XF (2001) Evaluation on the protein quality of Orychophragmus violaceus. Acta Boanica Boreali-Occidentalia Sinica 21:673–677
Whitlock MC, Mccauley DE (1999) Indirect measures of gene flow and migration: FST ≠ 1/(4Nm + 1). Heredity 82:117–125
Wolfe AD, Liston A (1998) Contributions of PCR-based methods to plant systematics and evolutionary biology. In: Soltis DE, Soltis PS, Doyle JJ (eds) Molecular systematics of plants II: DNA sequencing. Kluwer, New York, pp 43–86
Wu YY, Jiang JY, Shuai SW, Yao LZ (1996) A study on the cytogenetics of Orychophragmus violaceus. Southwest China J Agric Sci 3:38–41
Wu NF, Li RG, Wu XM, Zhu L, Fan YL, Qian XZ (1997) RAPD molecular markers and genetic diversity among 40 cultivars of Brassica napus in China. Chinese Biodiver 5(246):246–250
Wu YY, Wang B, Taylor PWJ (2002) Comparaison study on several molecular markers between rapeseed and Orychophragmus violaceus. Chinese J Oil Crop Sci 2:22–25
Xiao L, Luo P (1994) The research presence and development prospect of Orychophragmus violaceus. Acta Botanica Boreali-Occidentalia sinica 14:237–241
Yeh FC, Yang RC, Boyle T (1999) POPGENE Version 1.31, Microsoft window-based freeware for population genetic analysis. University of Alberta and Centre for International Forestry Research, Edmonton
Zhang LJ, Dai SL (2005) The value of development of Orychophragmus violaceus and its landscape utilization. Beijing Landscape 4:43–45
Zhang LJ, Qin HM, Wang M, Dai SL (2005) Variation of morphological characteristics of Orychophragmus violaceus. Biodivers Sci 13:535–545
Zhang LJ, Duan C, Dai SL (2006) A studies on karyotypes of four populations in Orychophragmus violaceus from Beijing, The 2006 doctoral forum of China, pp 2504–2512
Zhang LJ, Han Y, Dai SL (2007) The pollination biology of Orychophragmus violaceus. Northern Horticul 12:119–121
Zhao Y, Wang ML, Wang TJ (1995) Low temperature pollen preservation of Orychophragmus violaceus. Southwest China J Agric Sci 3:65–69
Zhao WG, Zhou ZH, Miao XX, Zhang Y, Wang SB, Huang JH, Xiang H, Pan YL, Huang YP (2007a) A comparison of genetic variation among wild and cultivated Morus species (Moraceae: Morus) as revealed by ISSR and SSR markers. Biodivers Conserv 16:275–290
Zhao Y, Chen XY, Wang XR, Pian RQ (2007b) ISSR analysis of genetic diversity among Lespedeza bicolor populations. J Plant Genetic Resour 2:195–199
Zhou TY, Guan KJ, Guo RL (1987) Flora Reipublicae Popularis Sinicae. Tomus 33. Science Press, Beijing, pp 40–43
Zhou JM, Wei ZM, Xu ZH, Liu SG, Luo P (1997) Agrobacterium-mediated transformation of Orychophragmus violaceus and regeneration of transgenic plants. Acta Phytophysiologica Sinica 23:21–28
Ziekiewica E, Ratalski A, Labuda D (1994) Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 20:176–183
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, LJ., Dai, SL. Genetic variation within and among populations of Orychophragmus violaceus (Cruciferae) in China as detected by ISSR analysis. Genet Resour Crop Evol 57, 55–64 (2010). https://doi.org/10.1007/s10722-009-9450-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-009-9450-2