Abstract
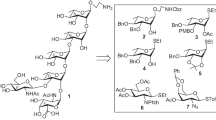
Synthesis of the pentasaccharide with a 2-aminoethyl linker attached to the reducing end corresponding to the cell wall O-antigen of Escherichia coli O86 strain is reported. The synthetic strategy involves sequential glycosylation of suitably protected monosaccharide intermediates under similar glycosylation reaction conditions. Thioglycosides have been used as glycosyl donor throughout the synthetic strategy. Conformational analysis of the synthesized pentasaccharide has been carried out using 2D ROESY NMR spectral analysis and all atom explicit molecular dynamics (MD) simulation technique.

Facile synthesis of the pentasaccharide with a 2-aminoethyl linker attached to the reducing end corresponding to the cell wall O-antigen of Escherichia coli O86 strain is reported. Conformational analysis of the synthesized pentasaccharide has been carried out using 2D ROESY NMR spectral analysis and all atom explicit molecular dynamics (MD) simulation technique.





Similar content being viewed by others
References
Andersson D.I., Hughes D.: Persistence of antibiotic resistance in bacterial populations. FEMS Microbiol. Rev. 35, 901–911 (2011)
Bhatia S., Dimde M., Haag R.: Multivalent glycoconjugates as vaccines and potential drug candidates. Med. Chem. Commun. 5, 862–878 (2014)
Källenius G., Pawlowski A., Hamasur B., Svenson S.B.: Mycobacterial glycoconjugates as vaccine candidates against tuberculosis. Trends Microbiol. 16, 456–462 (2008)
Lucas A.H., Apicella M.A., Taylor C.E.: Carbohydrate moieties as vaccine candidates. Clin. Infect. Dis. 41, 705–712 (2005)
Astronomo R.D., Burton D.R.: Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat. Rev. Drug Discov. 9, 308–324 (2010)
Lebeer S., Vanderleyden J., De Keersmaecker S.C.J.: Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat. Rev. Microbiol. 8, 171–184 (2010)
Roberts I.S., Roberts I.S.: The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu. Rev. Microbiol. 50, 285–315 (1996)
Raetz C.R.H., Whitfield C.: Lipopolysaccharide Endotoxins. Annu. Rev. Microbiol. 71, 635–700 (2002)
Nataro J.P., Kaper J.B.: Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11, 142–201 (1998)
Andersson M., Carlin N., Leontein K., Lindquist U., Slettengren K.: Structural studies of the O-antigenic polysaccharide of Escherichia coli O86, which possesses blood-group B activity. Carbohydr. Res. 185, 211–223 (1989)
Yi W., Bystricky P., Yao Q., Guo H., Zhu L., Li H., Shen J., Li M., Ganguly S., Allen Bush C., Wang P.G.: Two different O-polysaccharides from Escherichia coli O86 are produced by different polymerization of the same O-repeating unit. Carbohydr. Res. 341, 100–108 (2006)
Niqudkar S.S., Demchenko A.V.: Stereocontrolled 1,2-cis glycosylation as the driving force of progress in synthetic carbohydrate chemistry. Chemical Sc. 6, 2687–2704 (2015)
Ingle A.B., Chao C.-S., Hung W.-C., Tony Mong K.-K.: Chemical synthesis of the O-antigen repeating unit of Escherichia coli O86 by an N-formylmorpholine-modulated one-pot glycosylation strategy. Asian J. Org. Chem. 3, 870–876 (2014)
Si A., Misra A.K.: Expedient synthesis of the pentasaccharide repeating unit of the polysaccharide O-antigen of Escherichia coli O11. Chemistry Open. 5, 47–50 (2016)
Santra A., Ghosh T., Misra A.K.: Expedient synthesis of two structurally close tetrasaccharides corresponding to the O-antigens of Escherichia coli O127 and Salmonella enterica O13. Tetrahedron-Asymmetry. 23, 1385–1392 (2012)
Lönn H.: Synthesis of a tri- and a hepta-saccharide which contain α-L-fucopyranosyl groups and are part of the complex type of carbohydrate moiety of glycoproteins. Carbohydr. Res. 139, 105–113 (1985)
Ghosh S., Misra A.K.: Concise synthesis of a hexasaccharide present in the cell wall lipopolysaccharide of Azospirillum lipoferum Sp59b. Tetrahedron-Asymmetry. 21, 725–730 (2010)
Ghosh S., Misra A.K.: Concise synthesis of a hexasaccharide related to the adhesin receptor of Streptococcus oralis ATCC 55229. J. Carbohydr. Chem. 28, 447–462 (2009)
Mukherjee, C., Misra, A. K. Glycosylation and pyranose-furanose isomerization of carbohydrates using HClO4-SiO2: synthesis of oligosaccharides containing galactofuranose. Synthesis 683–692 (2007).
Chakraborti A.K., Gulhane R.: Perchloric acid adsorbed on silica gel as a new, highly efficient, and versatile catalyst for acetylation of phenols, thiols, alcohols, and amines. Chem. Commun. 2003, (1896-1897)
Zemplén G.: Abbau der reduzierenden Biosen, I.: Direkte konstitutions-ermittlung der cellobiose. Ber. Dtsch. Chem. Ges. 59, 1254–1266 (1926)
Agnihotri G., Misra A.K.: Mild and efficient method for the cleavage of benzylidene acetals using HClO4-SiO2 and direct conversion of acetals to acetates. Tetrahedron Lett. 47, 3653–3658 (2006)
Demchenko, A., Stauch, T., Boons, G.-J. Solvent and other effects on the stereoselectivity of thioglycoside glycosidations. Synlett 818–820 (1997).
Satoh H., Hansen H.S., Manabe S., van Gunsteren W.F., Hünenberger P.H.: Theoretical investigation of solvent effects on glycosylation reactions: stereoselectivity controlled by preferential conformations of the intermediate oxacarbenium-counterion complex. J. Chem. Theory Comput. 6, 1783–1797 (2010)
Ogawa T., Yamamoto H.: Synthesis of a model linear mannohexaose for the backbone structure of fruit body polysaccharide of Tremella fuciformis and Dictyophora indusiata FISCH. Agric. Biol. Chem. 49, 475–482 (1985)
Nakahara Y., Ogawa T.: Solid-phase synthesis of an O-linked glycopeptide based on a benzyl-protected glycan approach. Carbohydr. Res. 292, 71–81 (1996)
Santra A., Ghosh T., Misra A.K.: Removal of benzylidene acetal and benzyl ether in carbohydrate derivatives using triethylsilane and Pd/C. Beilstein J. Org. Chem. 9, 74–78 (2013)
Santra A., Si A., Kar R.K., Bhunia A., Misra A.K.: Linear synthesis and conformational analysis of the pentasaccharide repeating unit of the cell wall O-antigen of Escherichia coli O13. Carbohydr. Res. 391, 9–15 (2014)
Kar R.K., Suryadevara P., Sahoo B.R., Sahoo G.C., Dikhit M.R., Das P.: Exploring novel KDR inhibitors based on pharmaco-informatics methodology. SAR QSAR Environ. Res. 24, 215–234 (2013)
Jorgensen W.L., Chandrasekhar J., Madura J.D., Impey R.W., Klein M.L.: Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983)
Vincent K., van Gunsteren W.F., Hünenberger P.H.: A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. J. Comput. Chem. 22, 501–508 (2001)
Dhara D., Kar R.K., Bhunia A., Misra A.K.: Convergent synthesis and conformational analysis of the hexasaccharide repeating unit of the O-antigen of Shigella flexneri serotype 1d. Eur. J. Org. Chem. 2014(21), 4577–4584 (2014)
Kar R.K., Suryadevara P., Jana J., Bhunia A., Chatterjee S.: Novel G-quadruplex stabilizing agents: in-silico approach and dynamics. J. Biomol. Struct. Dyn. 31, 1497–1518 (2013)
Acknowledgments
I. B. and R. K. K. thank CSIR, India for providing Senior Research Fellowships respectively. This work was supported by SERB, New Delhi, India (AKM) [Project No. EMR/2015/000282]. The authors sincerely thank reviewers for their valuable comments to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 2869 kb)
Rights and permissions
About this article
Cite this article
Bhaumik, I., Kar, R.K., Bhunia, A. et al. Expedient synthesis of the pentasaccharide repeating unit of the O-antigen of Escherichia coli O86 and its conformational analysis. Glycoconj J 33, 887–896 (2016). https://doi.org/10.1007/s10719-016-9687-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-016-9687-x